Visible-Light-Induced NHC-Catalyzed Carboacylation Reaction of Alkenes from Aryl Thianthrenium Salts and Aldehydes
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A metal-free, visible-light-induced NHC-catalyzed multiple-component reaction involving aldehydes and aryl thianthrenium salts for the carboacylation reaction of alkenes is reported. In this reaction, NHC-activated aldehydes afforded Breslow intermediates, which reduced thianthrenium salts and generated aryl radicals. The resulting aryl radicals underwent radical addition reactions to yield arylacylation products, in the presence of iodoalkane, and participated in the halogen atom transfer process to generate alkyl radicals and facilitate olefin alkylacylation.
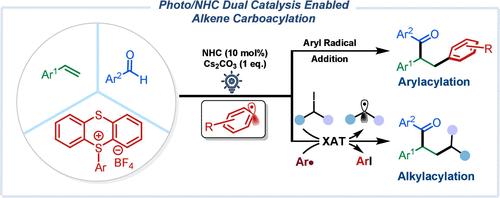
可见光诱导nhc催化芳基硫鎓盐和醛的烯基碳酰化反应
报告了一种无金属、可见光诱导的 NHC 催化多组分反应,该反应涉及醛和芳基噻吩鎓盐,用于烯烃的羧基化反应。在该反应中,NHC 活化的醛生成布雷斯洛中间体,布雷斯洛中间体还原噻吩盐并生成芳基自由基。在碘烷烃存在的情况下,生成的芳基发生自由基加成反应,生成芳基酰化产物,并参与卤原子转移过程,生成烷基自由基,促进烯烃烷基酰化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


