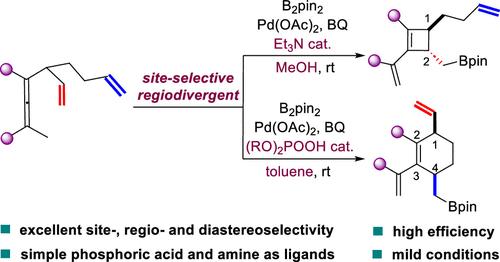
Palladium-Catalyzed Site-Selective Regiodivergent Carbocyclization of Di- and Trienallenes: A Switch between Substituted Cyclohexene and Cyclobutene
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nature efficiently produces a myriad of structurally diverse carbon ring frameworks from common linear precursors via cyclization reactions at specific olefinic sites in dienes or polyenes. In contrast, achieving the site-selective functionalization of diene or polyene substrates remains a formidable challenge in chemical synthesis. Herein, we report a pair of highly site-selective, regiodivergent carbocyclization reactions of dienallenes and trienallenes, enabling the efficient synthesis of cis-1,4-disubstituted cyclohexenes and trans-1,2-disubstituted cyclobutenes from a common precursor with high diastereoselectivity. Remarkably, simple achiral organophosphoric acids and amines are identified as powerful ligands for controlling these palladium-catalyzed regiodivergent carbocyclizations. This approach represents the first example of site-selective regiodivergent carbocyclization, providing a practical method for the stereospecific synthesis of thermodynamically disfavored cis-1,4-disubstituted cyclohexenes and fully substituted trans-1,2-cyclobutenes. Additionally, the methodology developed offers general insights into the development of metal-catalyzed site-selective, regiodivergent carbocyclizations of diene and polyene precursors, mimicking natural carbocyclization processes.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


