Direct Imaging of Chirality Transfer Induced by Glycosidic Bond Stereochemistry in Carbohydrate Self-Assemblies
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
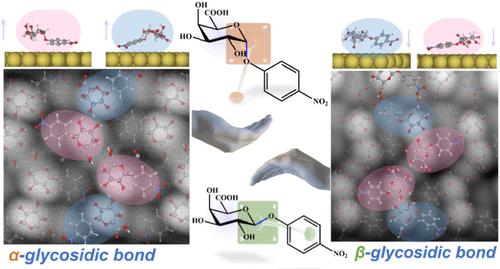
Carbohydrates, essential biological building blocks, exhibit functional mechanisms tied to their intricate stereochemistry. Subtle stereochemical differences, such as those between the anomers maltose and cellobiose, lead to distinct properties due to their differing glycosidic bonds; the former is digestible by humans, while the latter is not. This underscores the importance of precise structural determination of individual carbohydrate molecules for deeper functional insights. However, their structural complexity and conformational flexibility, combined with the high spatial resolution needed, have hindered direct imaging of carbohydrate stereochemistry. Here, we employ noncontact atomic force microscopy integrated with a data-efficient, multifidelity structure search approach accelerated by machine learning integration to determine the precise 3D atomic coordinates of two carbohydrate anomers on Au(111). We observe that the stereochemistry of the glycosidic bond regulates on-surface chiral selection in carbohydrate self-assemblies. The reconstructed models, validated against experimental data, provide reliable atomic-scale structural evidence, uncovering the origin of the on-surface chirality from carbohydrate anomerism. Our study confirms that nc-AFM is a reliable technique for real-space discrimination of carbohydrate stereochemistry at the single-molecule level, providing a pathway for bottom-up investigations into the structure–property relationships of carbohydrates in biological research and materials science.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


