Deconvolving lithium-ion redox in vanadium–iron oxide aerogels using X-ray absorption spectroscopy and density functional theory
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
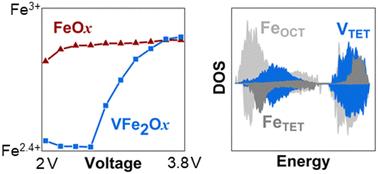
Substitution of vanadium into earth-abundant maghemite iron oxide introduces cation vacancies that increase Li+ storage capacity concomitant with a positive shift in its electrochemical potential. Expressing vanadium ferrite (VFe2Ox) as an aerogel offers an opportunity to probe Li+ storage in this inherently defective spinel from highly disordered (X-ray amorphous) to nanocrystalline. To understand the redox sequence of the host cations, we use in situ X-ray absorption near-edge spectroscopy (XANES) obtained using an in-lab X-ray absorption spectrometer in concert with density functional theory calculations to uncover the quantum mechanical-level effects that underpin relevant energy-storage behaviors. The Fe K-edge spectra indicate that upon Li+ insertion, the change in Fe oxidation state occurs primarily at high voltage (average voltage ∼2.9 V), which is ∼0.7 V higher than the average voltage for γ-Fe2O3. Parallel computations using density functional theory show that tetrahedral V and octahedral Fe sites are reduced during lithiation and that the hybridization of Fe and V orbitals imposes a positive shift in voltage for Fe redox. Our combined experimental and computational investigation sheds light on how these complex materials store Li+ and increase cell voltage. These findings point toward future compositional alterations that may further improve their properties.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


