GPNMB disrupts SNARE complex assembly to maintain bacterial proliferation within macrophages
IF 19.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Xenophagy plays a crucial role in restraining the growth of intracellular bacteria in macrophages. However, the machinery governing autophagosome‒lysosome fusion during bacterial infection remains incompletely understood. Here, we utilize leprosy, an ideal model for exploring the interactions between host defense mechanisms and bacterial infection. We highlight the glycoprotein nonmetastatic melanoma protein B (GPNMB), which is highly expressed in macrophages from lepromatous leprosy (L-Lep) patients and interferes with xenophagy during bacterial infection. Upon infection, GPNMB interacts with autophagosomal-localized STX17, leading to a reduced N-glycosylation level at N296 of GPNMB. This modification promotes the degradation of SNAP29, thus preventing the assembly of the STX17-SNAP29-VAMP8 SNARE complex. Consequently, the fusion of autophagosomes with lysosomes is disrupted, resulting in inhibited cellular autophagic flux. In addition to Mycobacterium leprae, GPNMB deficiency impairs the proliferation of various intracellular bacteria in human macrophages, suggesting a universal role of GPNMB in intracellular bacterial infection. Furthermore, compared with their counterparts, Gpnmbfl/fl Lyz2-Cre mice presented decreased Mycobacterium marinum amplification. Overall, our study reveals a previously unrecognized role of GPNMB in host antibacterial defense and provides insights into its regulatory mechanism in SNARE complex assembly.
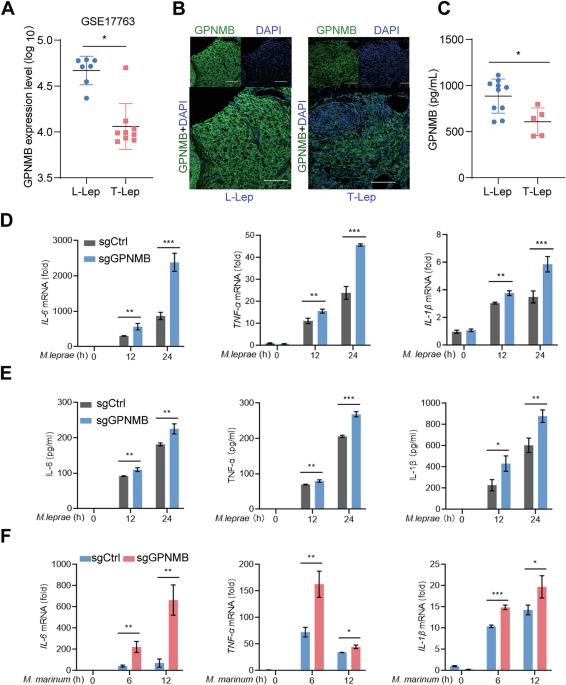
GPNMB破坏SNARE复合物组装以维持巨噬细胞内的细菌增殖。
巨噬细胞的异体噬噬在抑制细胞内细菌的生长中起着至关重要的作用。然而,在细菌感染过程中控制自噬体-溶酶体融合的机制仍然不完全清楚。在这里,我们利用麻风病,一个理想的模型来探索宿主防御机制和细菌感染之间的相互作用。我们强调了糖蛋白非转移性黑色素瘤蛋白B (GPNMB),该蛋白在麻风性麻风(L-Lep)患者的巨噬细胞中高度表达,并在细菌感染期间干扰异种吞噬。感染后,GPNMB与自噬体定位的STX17相互作用,导致GPNMB N296的n -糖基化水平降低。这种修饰促进了SNAP29的降解,从而阻止了STX17-SNAP29-VAMP8 SNARE复合物的组装。因此,自噬体与溶酶体的融合被破坏,导致细胞自噬通量受到抑制。除麻风分枝杆菌外,GPNMB缺乏还会损害人巨噬细胞中各种细胞内细菌的增殖,提示GPNMB在细胞内细菌感染中具有普遍作用。此外,与同类小鼠相比,Gpnmbfl/fl Lyz2-Cre小鼠的海洋分枝杆菌扩增率降低。总的来说,我们的研究揭示了GPNMB在宿主抗菌防御中以前未被认识到的作用,并为其在SNARE复合物组装中的调节机制提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


