A Library of Polymetallic Alloy Nanotubes: From Binary to Septenary
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
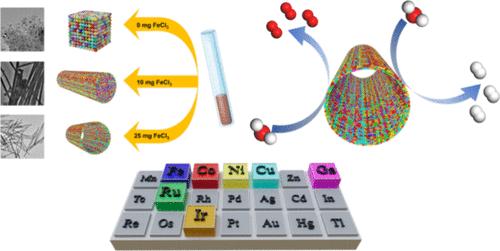
The polymetallic alloy nanostructure has received widespread attention in electrocatalytic reactions due to the variability in composition and excellent performance. However, the controllable synthesis of one-dimensional (1D) polymetallic alloy nanomaterials remains a significant challenge. Herein, we propose a new and general low-temperature method to prepare a library of binary to septenary polymetallic alloy nanotubes, and this method can be used to synthesize 18 kinds of polymetallic alloy nanotube (NT), including eight kinds of high-entropy alloy (HEA) NT. A representative Cu30Ni26Co19Ru14Ir11 HEA NT demonstrates efficient and stable catalytic performance for both oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). The Cu30Ni26Co19Ru14Ir11 HEA NT exhibits an overpotential of only 121 mV for the HER and 272 mV for the OER, respectively, when measured at a current density of 100 mA cm–2. In addition, the two-electrode system comprising the Cu30Ni26Co19Ru14Ir11 HEA NT exhibits an impressive efficiency of 100 mA cm–2 during overall water splitting, requiring only a potential of 1.67 V. The high catalytic activity of the Cu30Ni26Co19Ru14Ir11 HEA NT is attributed to the downward shift of the d-band center. In the HER, the downward shift of the d-band center can reduce the binding energy with *H, which is beneficial for the desorption process of hydrogen. In the OER, the downward shift can also reduce the reaction energy barrier associated with the rate-determining step from *O to *OOH. This work seeks to offer a new and general method for synthesizing polymetallic alloy nanotubes with controllable structures and compositions under mild conditions.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


