Visible-Light-Induced Divergent C–H Esterification/Alkylation of Quinoxalin-2(1H)-ones with Aldehydes under Mild Conditions
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
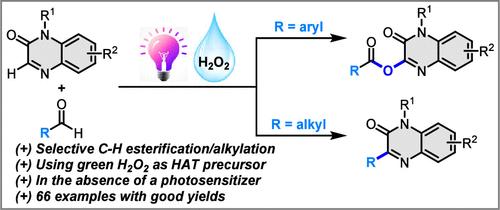
Herein, we introduce an efficient and straightforward strategy for the selective C–H esterification and alkylation of quinoxalin-2(1H)-ones with aldehydes. A key feature of our study is the ability to perform both C–H esterification and alkylation using different types of aldehydes. The reaction system is highly compatible with a range of quinoxalin-2(1H)-ones and aldehydes, yielding C3-esterified and C3-alkylated products in moderate-to-good yields. The applicability of this approach is further enhanced by its scalability through continuous-flow synthesis, late-stage modification of significant molecules, and product derivatization. Our mechanistic investigations reveal a radical relay mechanism, triggered by a hydrogen atom transfer process.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


