Transcription elongation factor ELOF1 is required for efficient somatic hypermutation and class switch recombination
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
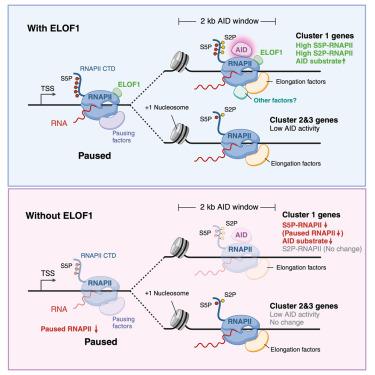
Somatic hypermutation (SHM) and class switch recombination (CSR) diversify immunoglobulin (Ig) genes and are initiated by the activation-induced deaminase (AID), a single-stranded DNA cytidine deaminase thought to engage its substrate during RNA polymerase II (RNAPII) transcription. Through a genetic screen, we identified numerous potential factors involved in SHM, including elongation factor 1 homolog (ELOF1), a component of the RNAPII elongation complex that functions in transcription-coupled nucleotide excision repair (TC-NER) and transcription elongation. Loss of ELOF1 compromises SHM, CSR, and AID action in mammalian B cells and alters RNAPII transcription by reducing RNAPII pausing downstream of transcription start sites and levels of serine 5 but not serine 2 phosphorylated RNAPII throughout transcribed genes. ELOF1 must bind to RNAPII to be a proximity partner for AID and to function in SHM and CSR, and TC-NER is not required for SHM. We propose that ELOF1 helps create the appropriate stalled RNAPII substrate on which AID acts.
转录延伸因子ELOF1是高效体细胞超突变和类开关重组所必需的
体细胞超突变(SHM)和类开关重组(CSR)使免疫球蛋白(Ig)基因多样化,并由激活诱导脱氨酶(AID)启动,AID是一种单链DNA胞苷脱氨酶,被认为在RNA聚合酶II (RNAPII)转录过程中与其底物结合。通过遗传筛选,我们确定了许多与SHM有关的潜在因素,包括延伸因子1同源物(ELOF1),它是RNAPII延伸复合体的一个组成部分,在转录偶联核苷酸切除修复(TC-NER)和转录延伸中起作用。ELOF1的缺失会影响哺乳动物B细胞中的SHM、CSR和AID作用,并通过减少RNAPII在转录起始位点下游的停顿和丝氨酸5(而非丝氨酸2)磷酸化RNAPII在转录基因中的水平,改变RNAPII的转录。ELOF1必须与RNAPII结合才能成为AID的邻近伙伴,并在SHM和CSR中发挥作用,而TC-NER不需要用于SHM。我们认为ELOF1有助于产生合适的停滞RNAPII底物,而AID则作用于其上。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


