Mass Transport-Dependent C–C Bond Formation for CO Electroreduction with Alkali Cations
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Electrolyte cation identity has been reported to influence the multicarbon (C2+) selectivity in CO2/CO electroreduction. However, most of the previous work for cation size effect is based on H-cell configurations, which may inadvertently distort the underlying mechanism of cation effect due to mass transport limitations, particularly for CO reduction. Here, using GDE-type flow electrolyzers, we report that the selectivity of total C2+ products on Cu is independent of alkali cation identity (Li+, Na+, K+, and Cs+) in the absence of the CO transport limitation. Notably, a high concentration of strongly hydrated cation (such as Li+) inhibits the total C2+ formation in CO reduction, whereas total C2+ selectivity is retained upon increasing concentrations of weakly hydrated cation (such as K+). Further investigations reveal that the CO coverage at a low cation concentration is almost independent of the cation identity, but the CO coverage at highly concentrated cations strongly relies on the alkali cation identity.
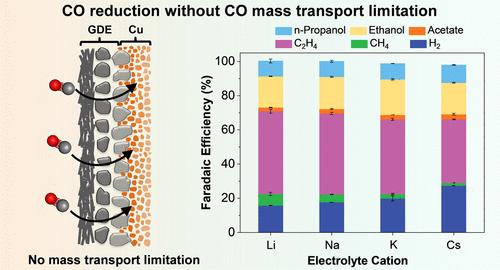
碱离子电还原CO的质量输运相关C-C键形成
电解质阳离子特性影响CO2/CO电还原过程中多碳(C2+)的选择性。然而,之前大多数关于阳离子尺寸效应的研究都是基于h电池的结构,由于质量传输的限制,这可能会无意中扭曲阳离子效应的潜在机制,特别是对于CO还原。在没有CO输运限制的情况下,使用gde型流动电解槽,我们报告了总C2+产物对Cu的选择性与碱阳离子(Li+, Na+, K+和Cs+)无关。值得注意的是,高浓度的强水合阳离子(如Li+)会抑制CO还原过程中总C2+的形成,而在增加弱水合阳离子(如K+)浓度时,总C2+的选择性仍保持不变。进一步的研究表明,低阳离子浓度下的CO覆盖几乎与阳离子身份无关,而高浓度阳离子下的CO覆盖则强烈依赖于碱阳离子身份。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


