Ferric Salt-Mediated Tough Zwitterionic Hydrogel
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
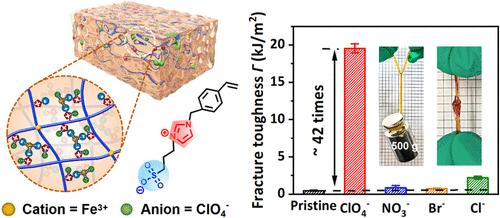
Polyzwitterionic gels possess a high density of ionic groups and present great potential in ionotronics. However, zwitterionic chains are easy to be highly hydrated, leading to weak interchain interactions and a poor mechanical performance. Although metal ions like Fe3+ show great effectiveness in toughening hydrogels, they usually weaken the zwitterionic gel inversely due to the anti-polyelectrolyte effect. Herein, a special co-ion effect of Fe(ClO4)3 is found, which is adopted to toughen the zwitterionic hydrogel. In contrast to the weak effect of Fe(NO3)3, FeBr3, and FeCl3 salts on gel toughness, Fe(ClO4)3 can toughen the imidazolium-based polyzwitterion (pVBIPS) effectively. The Fe(ClO4)3-treated pVBIPS gel exhibits a high ultimate tensile stress of 1.34 MPa, a Young’s modulus of 1.2 MPa, and tensile work of 2.29 MJ/m3, surpassing many existing pure zwitterionic hydrogels. It is found that the ClO4– ions can effectively bind to the imidazolium moiety of pVBIPS and promote the formation of SO3––Fe3+ interactions; the trivalent Fe3+ ions can then bridge multiple sulfonate groups to impart the strength. This work should offer a new strategy for developing tough zwitterionic hydrogels.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


