Molecular insight into the dynamics at the lithium-containing ionic liquid/gold film electrode interface using electrochemical attenuated total reflection spectroscopies
IF 2.9
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The spectral response at the interface between lithium-containing 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (EMIM-TFSI) and a gold electrode was investigated using electrochemical attenuated total reflection spectroscopy (EC-ATR) in the far-ultraviolet and infrared regions. At a negatively charged Au electrode within the cathodic limit, an increase in the EMIM cation signal and a decrease in the TFSI anion signal were observed for neat EMIM-TFSI, indicating the normal replacement of the TFSI anions by the EMIM cations. In contrast, an apparent decrease in the EMIM cation signal and an increase in the TFSI anion signal were observed, suggesting the replacement of the EMIM cation with a Li+ cation coordinated with TFSI anions. The ATR spectral responses were reversible in the electrode potential cycles, likely due to diffusion perpendicular to the electrode or the reorientation of the interfacial ionic liquid components. The surface-stabilized Li+ ions coordinated by the TFSI anions at the negatively charged Au electrode may restrict the direct interaction of the EMIM cation with the electrode, thereby reducing the reduction rate of the EMIM cation, and extending the cathodic limit upon the addition of the Li salt.
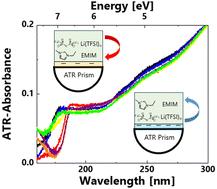
利用电化学衰减全反射光谱法(EC-ATR)研究了含锂的 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide (EMIM-TFSI) 与金电极界面在远紫外和红外区域的光谱响应。在阴极极限内的带负电金电极上,观察到纯 EMIM-TFSI 的 EMIM 阳离子信号增加,TFSI 阴离子信号减少,表明 EMIM 阳离子正常取代了 TFSI 阴离子。相反,观察到 EMIM 阳离子信号明显减少,TFSI 阴离子信号增加,这表明 EMIM 阳离子被与 TFSI 阴离子配位的 Li+ 阳离子取代。ATR 光谱响应在电极电位循环中是可逆的,这可能是由于垂直于电极的扩散或界面离子液体成分的重新定向。TFSI 阴离子在带负电的金电极上配位的表面稳定 Li+ 离子可能会限制 EMIM 阳离子与电极的直接相互作用,从而降低 EMIM 阳离子的还原率,并在添加锂盐后延长阴极极限。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Physical Chemistry Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
5.50
自引率
9.10%
发文量
2675
审稿时长
2.0 months
期刊介绍:
Physical Chemistry Chemical Physics (PCCP) is an international journal co-owned by 19 physical chemistry and physics societies from around the world. This journal publishes original, cutting-edge research in physical chemistry, chemical physics and biophysical chemistry. To be suitable for publication in PCCP, articles must include significant innovation and/or insight into physical chemistry; this is the most important criterion that reviewers and Editors will judge against when evaluating submissions.
The journal has a broad scope and welcomes contributions spanning experiment, theory, computation and data science. Topical coverage includes spectroscopy, dynamics, kinetics, statistical mechanics, thermodynamics, electrochemistry, catalysis, surface science, quantum mechanics, quantum computing and machine learning. Interdisciplinary research areas such as polymers and soft matter, materials, nanoscience, energy, surfaces/interfaces, and biophysical chemistry are welcomed if they demonstrate significant innovation and/or insight into physical chemistry. Joined experimental/theoretical studies are particularly appreciated when complementary and based on up-to-date approaches.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


