Construction of a catalyst using activated manganese ore and Co3O4 for efficient catalytic combustion of ethane†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
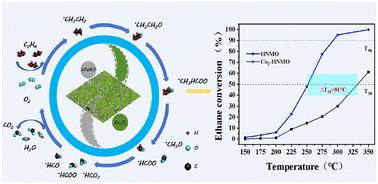
Natural manganese ore (NMO) has received extensive attention on account of its inherent stability, abundant reserves, and low cost. The higher specific surface area and abundant active sites are considered crucial for catalytic combustion. However, owing to the limited specific surface area inherent to natural manganese ore, we employed an acid etching activation treatment to enlarge the specific surface area of NMO (from 17.8 m2 g−1 to 48.9 m2 g−1). Herein, we successfully fabricated composite catalysts consisting of activated natural manganese ore (HNMO) and Co3O4 using the ethylene glycol sol–gel method and then evaluated them for the catalytic combustion of ethane. Results showed that the catalytic performance of the composite catalysts for ethane could not be enhanced monotonically by increasing the introduction of Co indiscriminately. Among them, the Co2-HNMO catalyst exhibited a higher specific surface area, increased Mn4+ and Co3+ contents and higher oxygen mobility, which were attributed to the significant interaction between Co3O4 and HNMO. The T90 of the corresponding Co2-HNMO catalyst was 294 °C under a space velocity of 40 000 mL g−1 h−1. This work presents new insights into the design and synthesis of efficient composite catalysts based on natural minerals.
用活性锰矿石和Co3O4构建乙烷†高效催化燃烧催化剂
天然锰矿因其固有的稳定性、丰富的储量和低廉的成本而受到广泛关注。较高的比表面积和丰富的活性位点被认为是催化燃烧的关键。然而,由于天然锰矿石固有的有限比表面积,我们采用酸蚀活化处理来扩大NMO的比表面积(从17.8 m2 g−1增加到48.9 m2 g−1)。本文采用乙二醇溶胶-凝胶法制备了由活性天然锰矿石(HNMO)和Co3O4组成的复合催化剂,并对其催化乙烷燃烧性能进行了评价。结果表明,不加选择地增加Co的引入量,不会单调地提高复合催化剂对乙烷的催化性能。其中,Co2-HNMO催化剂表现出更高的比表面积、Mn4+和Co3+含量的增加以及更高的氧迁移率,这是由于Co3O4与HNMO之间存在显著的相互作用。在40000 mL g−1 h−1空速下,相应的Co2-HNMO催化剂的T90为294℃。这项工作为基于天然矿物的高效复合催化剂的设计和合成提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


