Phenol[4]arenes: Excellent Macrocyclic Precursors for Constructing Chiral Porous Organic Cages
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
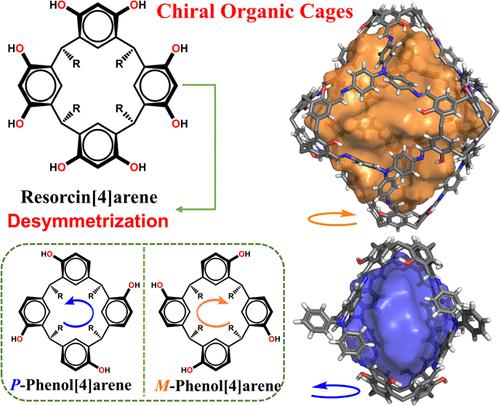
The development of new chiral building blocks for constructing complex chiral architectures, such as macrocycles and cages, is both crucial and challenging. Although concave-shaped calixarenes have been established as versatile building blocks for the synthesis of cage compounds, there are no reports on cages constructed from chiral calix[4]arene derivatives. Herein, we present a straightforward and effective method for gram-scale synthesis of a new member of chiral calix[4]arene macrocycle enantiomers, namely, phenol[4]arene (PC[4]A). As a proof of concept, we functionalized these enantiomers into tetraformylphenol[4]arene (PC[4]ACHO) derivatives via the Duff reaction to construct chiral porous organic cages (CPOCs) using polyamine synthons. Specifically, we employ two fluorescent amine synthons, bis(4-aminophenyl)phenylamine and tris(4-aminophenyl)amine, to assemble with PC[4]ACHO enantiomers, resulting in [2 + 4] lantern-shaped and [6 + 8] truncated octahedral CPOCs, respectively. These structures have been unambiguously characterized by single-crystal X-ray diffraction and circular dichroism (CD) spectroscopy. Notably, the [6 + 8] truncated CPOCs exhibit internal diameters of approximately 3.1 nm, a cavity volume of around 5300 Å3, and high specific surface areas of up to 1300 m2 g–1 after desolvation, making them among the largest CPOCs reported. Additionally, investigations into their chiral sensing performance demonstrate that these PC[4]A-based CPOCs enable the enantioselective recognition of amino acids and their derivatives. This work strongly suggests that PC[4]A can serve as an excellent building block for the rational design of chiral materials with practical applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


