Dual-activation-driven iodofunctionalization of electron-deficient alkenes: sulfonamidation, esterification, phosphorylation, and etherification†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-27
DOI:10.1039/d5qo00242g
引用次数: 0
Abstract
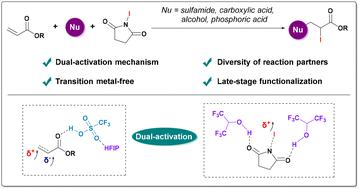
Iodofunctionalization of alkenes is a key strategy in synthetic chemistry but has traditionally been limited to electron-rich alkenes. Here, we present a versatile method for iodofunctionalizing electron-deficient alkenes, with an unprecedented substrate scope and the ability to incorporate a variety of nucleophiles, including sulfonamides, carboxylic acids, phosphoric acids, and alcohols. This versatile iodofunctionalization methodology relies on a dual-activation strategy that combines trifluoromethanesulfonic acid (TfOH) and hexafluoroisopropanol (HFIP). We demonstrate the scalability of this method through gram-scale synthesis and late-stage modification of complex products, underscoring its practical applicability. Mechanistic experiments and density functional theory (DFT) calculations provide compelling evidence that the dual-activation mode is critical for the efficient transformation.
缺电子烯的双活化驱动碘官能化:磺酰胺化、酯化、磷酸化和醚化
烯烃的碘官能化是合成化学中的一个关键策略,但传统上仅限于富电子烯烃。在这里,我们提出了一种用于碘功能化缺电子烯烃的通用方法,具有前所未有的底物范围和结合各种亲核试剂的能力,包括磺酰胺、羧酸、磷酸和醇。这种通用的碘功能化方法依赖于结合三氟甲烷磺酸(TfOH)和六氟异丙醇(HFIP)的双激活策略。我们通过克级合成和复杂产品的后期修饰证明了这种方法的可扩展性,强调了它的实用性。力学实验和密度泛函理论(DFT)计算提供了令人信服的证据,证明双激活模式是有效转换的关键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


