Efficient Metabolomics Profiling from Plasma Extracellular Vesicles Enables Accurate Diagnosis of Early Gastric Cancer
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Accurate diagnosis of early gastric cancer is valuable for asymptomatic populations, while current endoscopic examination combined with pathological tissue biopsy often encounters bottlenecks for early-stage cancer and causes pain to patients. Liquid biopsy shows promise for noninvasive diagnosis of early gastric cancer; however, it remains a challenge to achieve accurate diagnosis due to the lack of highly sensitive and specific biomarkers. Herein, we propose a protocol combining metabolomics profiling from plasma extracellular vesicles (EVs) and machine learning to identify the metabolomics discrepancies of early gastric cancer individuals from other populations. Efficient metabolomics profiling is achieved by efficient, high-purity, and damage-free plasma EVs separation using elaborately designed nanotrap-structured microparticles (NanoFisher) by taking advantage of stereoscopic interaction and affinity interaction. Significant metabolomics discrepancies are obtained from 150 early gastric cancer (50), benign gastric disease (50), and non-disease control (50) plasma samples. Machine learning enables ideal distinction between early gastric cancer and non-disease control samples with an area under the curve (AUC) of 1.000, achieves an AUC of 0.875–0.975 for differentiating early gastric cancer from benign gastric diseases, and demonstrates an overall accuracy of 92% in directly classifying these three categories. The plasma EV metabolomics profiling enabled by NanoFisher materials, integrated with machine learning, holds considerable promise for broad clinical acceptance, enhancing gastric cancer screening outcomes.
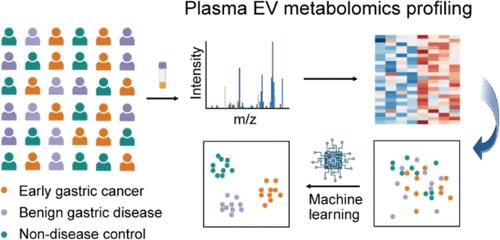
血浆细胞外囊泡的高效代谢组学分析有助于准确诊断早期胃癌
早期胃癌的准确诊断对无症状人群非常重要,而目前的内窥镜检查结合病理组织活检往往会遇到早期癌症的瓶颈,给患者带来痛苦。液体活检有望实现早期胃癌的无创诊断,但由于缺乏高灵敏度和特异性的生物标记物,要实现准确诊断仍是一项挑战。在此,我们提出了一种结合血浆细胞外囊泡(EVs)代谢组学分析和机器学习的方案,以确定早期胃癌患者与其他人群的代谢组学差异。使用精心设计的纳米捕获器结构微粒(NanoFisher),利用立体相互作用和亲和力相互作用的优势,高效、高纯度、无损伤地分离血浆EVs,从而实现高效的代谢组学分析。150 份早期胃癌(50 份)、良性胃病(50 份)和非疾病对照(50 份)血浆样本的代谢组学差异显著。机器学习能理想地区分早期胃癌和非疾病对照样本,曲线下面积(AUC)为 1.000,区分早期胃癌和良性胃病的 AUC 为 0.875-0.975,在直接分类这三类样本时的总体准确率为 92%。由 NanoFisher 材料实现的血浆 EV 代谢组学分析与机器学习相结合,有望获得广泛的临床认可,提高胃癌筛查结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


