Bromination-Enhanced Chiral Interactions for Triphenylamine on Au and Ag(111)
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Controlled intermolecular steric interactions within self-assembled structures of chiral molecules are crucial for developing future spintronics and polarization devices. Here, we present that chiral-ordered assembly of brominated triphenylamine (TBPA) on Au(111) and Ag(111) surfaces is achieved by bromination-enhanced interactions between neighboring molecules, which has been characterized by scanning tunneling microscopy. For TBPA, the propeller-shaped conformation gives rise to two chiral enantiomers. In the self-assembled structures of TBPA formed on both Au(111) and Ag(111), we find that the chiral recognition is attributed to the stereoselective interactions between neighboring molecules, which are enhanced by the attractive bromophenyl–bromophenyl interactions. We also discuss the influence of substrate surfaces on the chiral-ordered assembly of TBPA. This study provides valuable insights into the role of bromo substituents in controlling the steric interactions of chiral molecules.
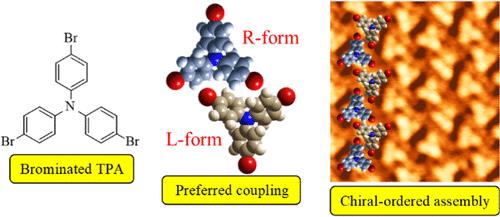
手性分子自组装结构中可控的分子间立体相互作用对于开发未来的自旋电子学和极化设备至关重要。在这里,我们介绍了溴化三苯胺(TBPA)在金(111)和银(111)表面上的手性有序组装是通过邻近分子间溴化增强的相互作用实现的,并通过扫描隧道显微镜对其进行了表征。对于 TBPA 而言,螺旋桨形构象产生了两种手性对映体。在金(111)和银(111)上形成的 TBPA 自组装结构中,我们发现手性识别归因于相邻分子之间的立体选择性相互作用,而这种相互作用又因具有吸引力的溴苯-溴苯相互作用而得到增强。我们还讨论了基底表面对 TBPA 手性有序组装的影响。这项研究为了解溴取代基在控制手性分子立体相互作用方面的作用提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


