Charge-Transfer Coefficient in the Kinetics of Single- and Multi-electron Transfer Redox Reactions
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
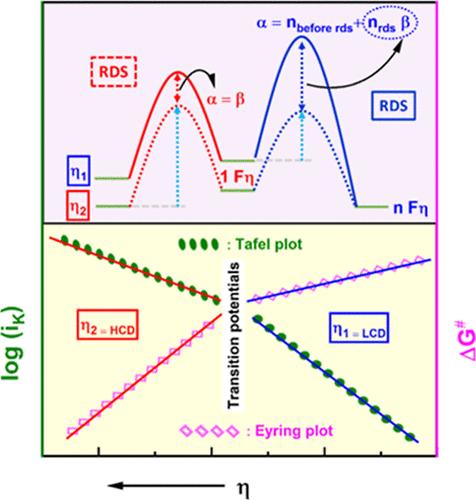
Single-step single-electron and multi-step multi-electron transfer reactions in neutral and acidic media, respectively, are investigated using a rotating-disk electrode (RDE) assembly as a function of temperature. The estimated kinetic current (ik) is analyzed using the classical Butler–Volmer (B–V) equation for single-step single-electron transfer reactions and the generalized B–V equation for multi-step multi-electron transfer reactions. The ik estimated as a function of temperature at very small intervals of overpotential (η) is used to determine the apparent activation enthalpy (ΔH#) and the pre-exponential factor (Af) (containing the apparent activation entropy (ΔS#)) from the Eyring analysis. The trends in the ΔH# and Af with η are analyzed. The plots of ΔH# and Af as a function of η exhibit the same number of slopes as that of the Tafel plots, corresponding to either a change in the rate-determining step (rds) or a change in the fractional coverage by the adsorbed intermediates in the kinetically operable overpotential range. Consequently, the estimated symmetry factor (β) or charge-transfer coefficient (α) values from both the Tafel and Eyring analyses reach a general consensus and explain the α values greater than 1 for multi-step multi-electron transfer processes. From the η dependence of ΔH# and ΔS#, the enthalpic and entropic components of β and α are estimated. Such analysis enhances the understanding of the significance of β and α, aiding the evaluation of the kinetic parameters, the interpretation of the proposed reaction mechanism, and the identification of rds.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


