Tailoring Octahedron-Tetrahedron Synergism in Spinel Catalysts for Acidic Water Electrolysis
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
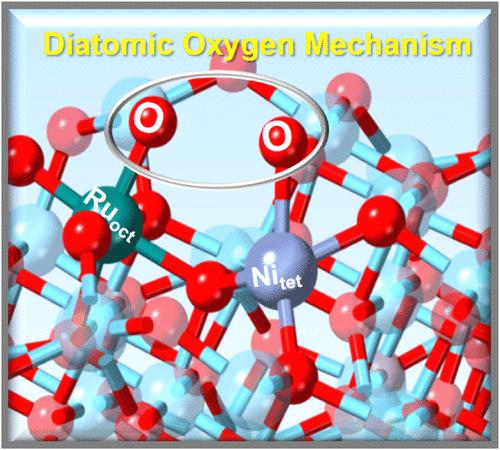
The instability issues of oxide-based electrocatalysts during the oxygen evolution reaction (OER) under acidic conditions, caused by the oxidation and dissolution of the catalysts along with the current-capacitance effect, constrain their application in proton exchange membrane water electrolysis (PEMWE). To address these challenges, we tailored the spinel structure of Co3O4 and exploited the synergism between the tetrahedron and octahedron sites by partially substituting Co with Ni and Ru (denoted as NiRuCoOx), respectively. Such a catalyst design creates a Ru–O–Ni electronic coupling effect, facilitating a direct dioxygen radical-coupled OER pathway. Density-functional theory (DFT) calculations and in situ Raman spectroscopy results confirm that Ru is the active site in the diatomic oxygen mechanism while Ni stabilizes lattice oxygen and the Ru–O bonding. The designed NiRuCoOx catalyst exhibits an exceptionally low overpotential of 166 mV to achieve a current density of 10 mA cm–2. Moreover, when serving as the anode in PEMWE, the NiRuCoOx requires 1.72 V to reach a current density of 3A cm–2, meeting the 2026 target set by the U.S. Department of Energy (DOE: 1.8 V@3A cm–2). The PEMWE device can operate stably for more than 1500 h with a significantly reduced performance decay rate of 0.025 mV h–1 compared to commercial RuO2 (2.13 mV h–1). This work provides an efficient method for tailoring the octahedron-tetrahedron sites of spinel Co3O4, which significantly improves the activity and stability of PEMWE.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


