Inhibiting Overoxidation of Dynamically Evolved RuO2 to Achieve a Win–Win in Activity–Stability for Acidic Water Electrolysis
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Proton exchange membrane (PEM) water electrolysis offers an efficient route to large-scale green hydrogen production, in which the RuO2 catalyst exhibits superior activity but limited stability. Unveiling the atomic-scale structural evolution during operando reaction conditions is critical but remains a grand challenge for enhancing the durability of the RuO2 catalyst in the acidic oxygen evolution reaction (a-OER). This study proposes an adaptive machine learning workflow to elucidate the potential-dependent state-to-state global evolution of the RuO2(110) surface within a complex composition and configuration space, revealing the correlation between structural patterns and stability. We identify the active state with distorted RuO5 units that self-evolve at low potential, which exhibits minor Ru dissolution and an activity self-promotion phenomenon. However, this state exhibits a low potential resistance capacity (PRC) and evolves into inert RuO4 units at elevated potential. To enhance PRC and mitigate the overevolution of the active state, we explore the metal doping engineering and uncover an inverse volcano-type doping rule: the doped metal–oxygen bond strength should significantly differ from the Ru–O bond. This rule provides a theoretical framework for designing stable RuO2-based catalysts and clarifies current discrepancies regarding the roles of different metals in stabilizing RuO2. Applying this rule, we predict and confirm experimentally that Na can effectively stabilize RuO2 in its active state. The synthesized Na–RuO2 operates in a-OER for over 1800 h without any degradation and enables long-term durability in PEM electrolysis. This work enhances our understanding of the operando structural evolution of RuO2 and aids in designing durable catalysts for a-OER.
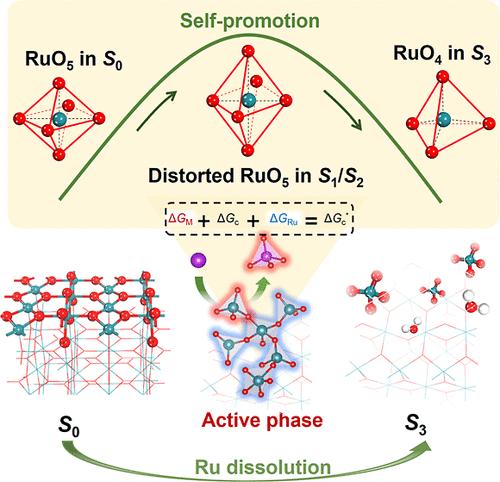
抑制动态生成的RuO2的过氧化,实现酸性电解活性与稳定性的双赢
质子交换膜(PEM)水电解为大规模绿色制氢提供了有效途径,其中RuO2催化剂表现出优异的活性,但稳定性有限。揭示在operando反应条件下的原子尺度结构演变是至关重要的,但对于提高RuO2催化剂在酸性析氧反应(a- oer)中的耐久性仍然是一个巨大的挑战。本研究提出了一种自适应机器学习工作流程,以阐明RuO2(110)表面在复杂组成和构型空间中潜在依赖的状态到状态的全局演化,揭示结构模式与稳定性之间的相关性。我们发现,在低电位下自进化的扭曲的RuO5单元具有活性态,表现出轻微的Ru溶解和活性自我促进现象。然而,这种状态表现出低电位电阻容量(PRC),并在高电位下演变成惰性的RuO4单元。为了增强PRC和减轻活性态的过度演化,我们探索了金属掺杂工程,发现了一个逆火山型掺杂规律:掺杂的金属-氧键强度应该与Ru-O键有显著差异。这一规律为设计稳定的RuO2基催化剂提供了理论框架,并澄清了目前不同金属在稳定RuO2中作用的差异。应用这一规律,我们预测并实验证实了Na能有效地稳定RuO2的活性态。合成的Na-RuO2在a-OER中工作超过1800小时,没有任何降解,并且在PEM电解中具有长期耐久性。本研究提高了我们对RuO2的operando结构演变的理解,并有助于设计耐用的a-OER催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


