Mechanism of formation of chiral allyl SCF3 compounds via selenium-catalyzed sulfenofunctionalization of allylboronic acids†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-27
DOI:10.1039/d4qo02170c
引用次数: 0
Abstract
The detailed reaction mechanism of diphenyl selenide-catalyzed sulfenofunctionalization of chiral α-CF3 allylboronic acids is investigated by means of density functional theory calculations. It is demonstrated that the reaction starts with the transfer of the SCF3 group from the (PhSO2)2NSCF3 reagent to the Ph2Se catalyst, a process that is shown to be assisted by the presence of Tf2NH acid. After a proton transfer step, the SCF3 group is transferred to the CC double bond of the substrate to generate a thiiranium ion. Concerted deborylative opening of the thiiranium ion yields then the final product. Several representative substrates are considered by the calculations, and the origins of the stereoselectivity of the reactions are analyzed by comparing the energies and geometries of the transition states leading to the different products.
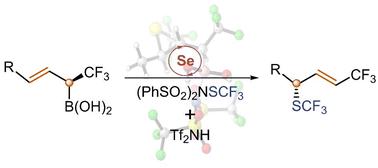
硒催化烯丙基硼酸磺酰基功能化手性烯丙基SCF3化合物的形成机理
采用密度泛函理论计算方法,研究了二苯基硒催化手性α-CF3烯基硼酸的磺胺官能化反应机理。实验证明,反应开始于SCF3基团从(PhSO2)2NSCF3试剂转移到ph2催化剂,这一过程被证明是由Tf2NH酸的存在所辅助的。经过质子转移步骤,SCF3基团被转移到底物的C=C双键上,生成硫鎓离子。协调的硫代铵离子的解旋开产生最终产物。计算中考虑了几种具有代表性的底物,并通过比较生成不同产物的过渡态的能量和几何形状,分析了反应立体选择性的来源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


