Acidochromic Behaviors of Indacenodithiophene-Based Conjugated Polymers Containing Azo, Imine, and Vinyl Bonds
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
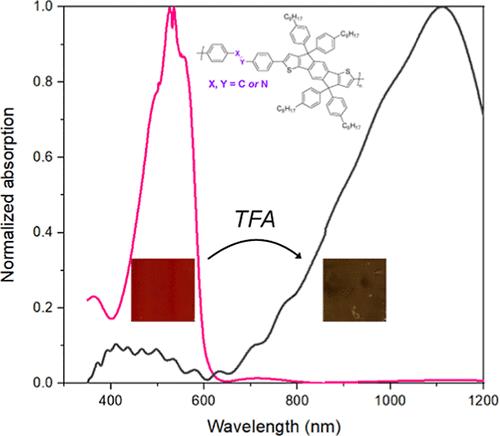
Acidochromic materials possess significant potential for the development of molecular switches, acid sensors, smart displays, and erasable/reprintable media. The semiconductive nature of conjugated polymers exhibiting such a behavior makes them ideal for use in electronic devices. In this study, we present a comparative investigation of three indacenodithiophene-based conductive polymers, containing azo, imine, and vinyl bonds (namely, PIDT-BAB, PIDT-BIB, and PIDT-BVB, respectively). We examined the alterations in the spectral properties of these polymers upon exposure to trifluoroacetic acid (TFA). The acidochromic response of PIDT-BAB and PIDT-BIB is indicated by DFT calculations to occur via protonation at the nitrogen atom. PIDT-BIB demonstrated heightened sensitivity to TFA. Conversely, PIDT-BVB did not display acidochromic properties in the film but was responsive to TFA in solution through acid doping. Repeated exposure of polymer films was used to examine the robustness of the polymers over 50 cycles. DFT calculations showed an increase in the planarity of PIDT-BAB and PIDT-BIB backbones as a result of protonation. This effect was particularly strong in PIDT-BAB, resulting in an unusually large bathochromic shift of 510 nm. The corresponding pink-to-transparent transition is particularly interesting for applications in sensors. Our findings provide valuable guidelines for the design of conjugated polymers tailored for acidochromic devices.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


