Leveraging Galvanic Exchange for the Formation of Shaped Metal Nanoparticles Comprising Non-Noble Metals
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
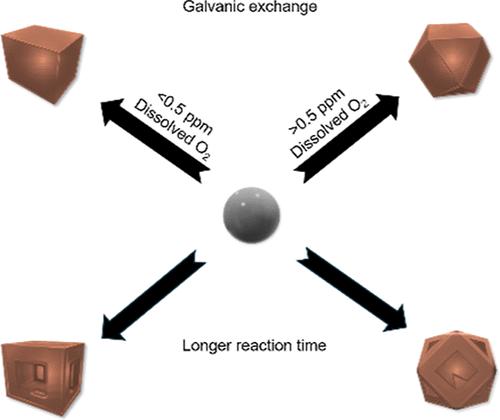
Colloidally synthesized nanoparticles are typically composed of noble metals due to their ease of use, which limits the development of tailorable catalytic materials. Non-noble metals are employed effectively as catalysts at the industrial scale, and their inclusion in nanoscale catalyst development has the potential to expand relevant pairings significantly. The inclusion of non-noble, crustally abundant metals, however, presents unique challenges including self-oxidation and negative reduction potentials, which limits the utility of conventional synthetic methods. Fundamental insights are critical to the expansion of available metals used in the formation of nanoparticles to include non-noble metals. This work highlights two key protocols for the synthesis of monodisperse spherical nickel/nickel oxide nanoparticles of tailorable sizes and subsequent transformation via galvanic exchange with copper ions to produce cubic and cuboctahedral copper oxide nanoparticles as well as controllable methods for the formation of bimetallic nickel–copper nanostructures. This innovative approach takes place in nanopure water at room temperature with no surfactants as a result of the thermodynamically favorable conditions. Additionally, the formation of shaped nanoparticles from a spherical nanoparticle via galvanic exchange is significantly different from that of known syntheses. This work provides critical insights into the strategic coupling of non-noble metals and elucidates a method to leverage galvanic exchange reactions in non-noble metal systems.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


