Photo- and Electrocatalytic Dual-Layer Cathode Facilitating Zn Peroxide Chemistry in Near-Neutral Zn–Air Batteries
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Rechargeable zinc-air batteries (ZABs) using near-neutral aqueous electrolytes are gaining significant attention due to their high energy density, low cost, high safety, and the excellent reversibility of the zinc (Zn) anode in mild electrolytes. However, the sluggish O2/ZnO2 conversion in the carbon-based cathodes of these batteries leads to a large voltage hysteresis (>600 mV) between charge and discharge. Metal- or metal oxide-based electrocatalysts are rarely used to reduce the overpotentials of this conversion because their presence may trigger undesirable H2O-participated oxygen reduction/evolution reactions, disrupting the pH balance of the electrolyte. Here, we propose a dual-layer catalytic cathode comprising an outer photocatalyst layer (exposed to air) of gold (Au) nanoparticles (NPs) decorated tungsten oxide (Au@WO3) loaded on carbon paper, and an inner electrocatalyst layer (exposed to the electrolyte) based on carbon nanotube (CNT). The hydrophobic inner CNT layer not only provides numerous active sites and ample accommodation for O2/ZnO2 conversion but also prevents the electrolyte from contacting the outer photocatalyst layer. Under light, the outer photocatalyst layer effectively separates photogenerated electron–hole pairs, which are then transferred to the inner CNT layer, reducing the overpotential of the O2/ZnO2 electrochemical conversion. As a result, the near-neutral ZAB demonstrates high stability at 0.1 mA cm–2; with a very small voltage hysteresis (<150 mV), significantly improving energy efficiency.
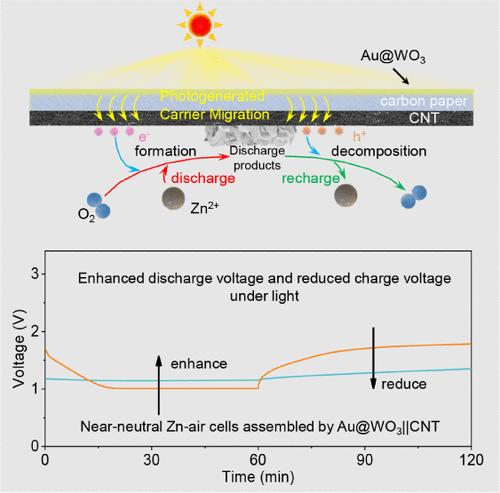
光催化和电催化双层阴极促进近中性锌空气电池中锌的过氧化化学
使用近中性水电解质的可充电锌空气电池(ZABs)因其高能量密度、低成本、高安全性以及锌(Zn)阳极在温和电解质中的优异可逆性而受到广泛关注。然而,这些电池的碳基阴极中O2/ZnO2转换缓慢,导致充电和放电之间的电压滞后较大(>600 mV)。金属或金属氧化物基电催化剂很少用于降低这种转化的过电位,因为它们的存在可能引发不希望的h2o参与的氧还原/析出反应,破坏电解质的pH平衡。在这里,我们提出了一种双层催化阴极,包括外部光触媒层(暴露在空气中),由负载在碳纸上的金(Au)纳米颗粒(NPs)装饰的氧化钨(Au@WO3)组成,以及内部电触媒层(暴露在电解质中),基于碳纳米管(CNT)。疏水的内层碳纳米管不仅为O2/ZnO2转化提供了大量的活性位点和充足的容纳空间,而且还阻止了电解质接触外光触媒层。在光照下,外层光催化剂层有效地分离光生成的电子空穴对,然后将其转移到内层碳纳米管层,从而降低了O2/ZnO2电化学转换的过电位。结果表明,接近中性的ZAB在0.1 mA cm-2下具有很高的稳定性;具有非常小的电压滞后(<150 mV),显著提高了能效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


