Functional Design of Peptide Materials Based on Supramolecular Cohesion
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
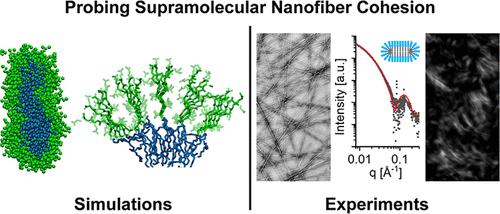
Peptide materials offer a broad platform to design biomimetic soft matter, and filamentous networks that emulate those in extracellular matrices and the cytoskeleton are among the important targets. Given the vast sequence space, a combination of computational approaches and readily accessible experimental techniques is required to design peptide materials efficiently. We report here on a strategy that utilizes this combination to predict supramolecular cohesion within filaments of peptide amphiphiles, a property recently linked to supramolecular dynamics and consequently bioactivity. Using established coarse-grained simulations on 10,000 randomly generated peptide sequences, we identified 3500 likely to self-assemble in water into nanoscale filaments. Atomistic simulations of small clusters were used to further analyze this subset of sequences and identify mathematical descriptors that are predictive of intermolecular cohesion, which was the main purpose of this work. We arbitrarily selected a small cohort of these sequences for chemical synthesis and verified their fiber morphology. With further characterization, we were able to link the latent heat associated with fiber to micelle transitions, an indicator of cohesion and potential supramolecular dynamicity within the filaments, to calculated hydrogen bond densities in the simulation clusters. Based on validation from in situ synchrotron X-ray scattering and differential scanning calorimetry, we conclude that the phase transitions can be easily observed by very simple polarized light microscopy experiments. We are encouraged by the methodology explored here as a relatively low-cost and fast way to design potential functions of peptide materials.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


