FAK inhibition combined with the RAF-MEK clamp avutometinib overcomes resistance to targeted and immune therapies in BRAF V600E melanoma
IF 48.8
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
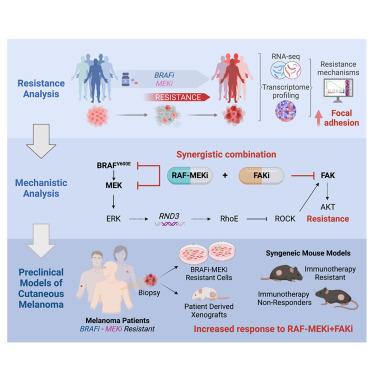
Widespread BRAF mutations result in persistent RAS-RAF-MEK-ERK (MAPK) signaling in melanoma. BRAF (BRAFi) and MEK (MEKi) inhibitors are approved for BRAF V600E melanomas, including those progressing on immunotherapy; however, rapid resistance to these agents highlights the need for novel strategies. Here, transcriptome analysis of BRAF V600E melanomas from patients resistant to BRAFi and MEKi shows activation of focal adhesion signaling. Consistently, BRAFi, MEKi, and the RAF-MEK clamp avutometinib activate focal adhesion kinase (FAK) in melanoma cells. Mechanistically, inhibition of an MAPK-RhoE (RND3) feedback loop results in the adaptive activation of RhoA-FAK-AKT. In turn, FAK inhibitors (FAKi) exert potent pro-apoptotic activity when combined with MAPK pathway inhibition. FAKi plus avutometinib overcomes resistance in multiple models derived from BRAFi plus MEKi-resistant melanoma patients and immunotherapy-resistant syngeneic mouse models. These findings provide a rationale for the development of avutometinib in combination with FAKi for patients with BRAF V600E melanoma progressing on BRAFi plus MEKi or immunotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


