Identification and Quantification of Al Pairs and Their Impact on Dealumination in Zeolites
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
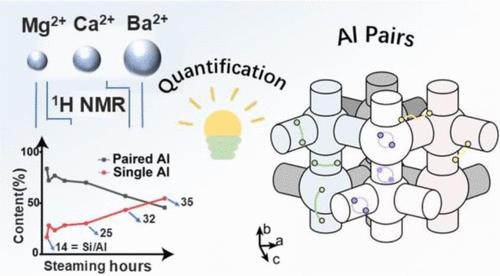
Understanding the precise quantity and spatial distribution of paired aluminum (Al) sites in zeolite catalysts is crucial, as they significantly impact the catalytic performance via synergistic effects and long-term stability. In this study, a novel strategy by employing divalent cation titration with varying cation sizes, in combination with advanced quantitative 1H NMR and 1H–1H homonuclear correlation techniques, has been developed to accurately identify and classify three distinct types of Al pairs. These include two types of Al pairs aligned along six-membered rings (6-MRs) and 10-membered rings (10-MRs), the latter of which are essentially composed of Al atoms located in different 6-MR or 5-MR. The third type comprises two Al atoms located in different channels. The second and third types had been challenging to probe in the past, yet they may be critical for catalysis, particularly the second type demonstrating proximity close enough to accommodate Ba2+ (with a radius of 1.49 Å). Our strategy for quantifying each type of Al pair marks a significant advancement in the understanding of the zeolite framework. Furthermore, controlled hydrothermal treatments using stepwise steaming reveal that a higher concentration of Al pairs accelerates dealumination, primarily for dynamic reasons of water molecules but not intrinsic structural instability induced by Al pairs. To address this, we propose a “bi-Al” vs “mono-Al” hydrolysis model, offering fresh insights into the pivotal role of Al pairs on zeolite stability. This work opens new avenues for optimizing zeolite-based catalysts for enhanced performance and longevity.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


