Pcf11/Spt5 condensates stall RNA polymerase II to facilitate termination and piRNA-guided heterochromatin formation
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The PIWI-interacting RNA (piRNA) pathway plays a crucial role in protecting animal germ cells by repressing transposons. However, the mechanism of piRNA-guided heterochromatin formation and its relationship to transcriptional termination remains elusive. Through RNA interference screening, we discovered Pcf11 and PNUTS as essential for piRNA-guided silencing in Drosophila germ line. Enforced tethering of Pcf11 leads to co-transcriptional repression and RNA polymerase II (RNA Pol II) stalling, and both are dependent on an α-helical region of Pcf11 capable of forming condensates. An intrinsically disordered region can substitute for the α-helical region of Pcf11 in its silencing capacity and support animal development, arguing for a causal relationship between phase separation and Pcf11’s function. Pcf11 stalls RNA Pol II by preferentially forming condensates with the unphosphorylated Spt5, promoted by the PP1/PNUTS phosphatase during termination. We propose that Pcf11/Spt5 condensates control termination by decelerating polymerase elongation, a property exploited by piRNAs to silence transposons and initiate RNA-mediated heterochromatin formation.
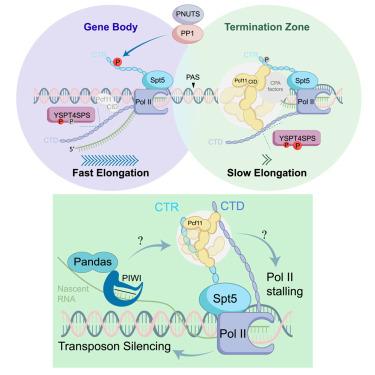
Pcf11/Spt5凝聚阻滞RNA聚合酶II,促进终止和pirna引导的异染色质形成
piwi相互作用RNA (piRNA)通路通过抑制转座子在保护动物生殖细胞中发挥重要作用。然而,pirna引导的异染色质形成的机制及其与转录终止的关系尚不清楚。通过RNA干扰筛选,我们发现Pcf11和PNUTS在果蝇种系中对pirna引导的沉默至关重要。强制捆绑Pcf11导致共转录抑制和RNA聚合酶II (RNA Pol II)停滞,两者都依赖于Pcf11能够形成凝聚物的α-螺旋区。一个内在无序的区域可以替代Pcf11的α-螺旋区,具有沉默能力并支持动物发育,这表明相分离与Pcf11的功能之间存在因果关系。Pcf11通过优先与未磷酸化的Spt5形成凝聚物来阻止RNA Pol II, PP1/PNUTS磷酸酶在终止过程中促进了这一过程。我们提出Pcf11/Spt5凝聚物通过减缓聚合酶延伸来控制终止,pirna利用这一特性沉默转座子并启动rna介导的异染色质形成。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


