Asymmetric Total synthesis of Asperones A and B through Organocatalyzed Quinone [5 + 2] Cycloaddition
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of the American Chemical Society
Pub Date : 2025-02-13
DOI:10.1021/jacs.4c1625210.1021/jacs.4c16252
引用次数: 0
Abstract
The first asymmetric total synthesis of the anti-inflammatory polyketides asperones A (1) and B (2) has been accomplished. Key synthetic steps include a Diels–Alder and retro-Diels–Alder cascade to construct the poly substituted phenol, an Al-Salen-catalyzed asymmetric cyanosilylation to form the tertiary alcohol of gregatin A, and an organocatalyzed intermolecular [5 + 2] cycloaddition of p-quinone with electron-deficient alkenes to build the crucial [3.2.1] octane core of asperones A (1) and B (2).
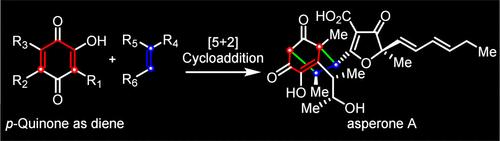
有机催化醌[5 + 2]环加成不对称合成asperone A和B
首次完成了抗炎多酮类化合物asperones A(1)和B(2)的不对称全合成。关键的合成步骤包括Diels-Alder级联和反Diels-Alder级联构建多取代苯酚,al - salen催化的不对称氰硅化反应形成gregatin a的叔醇,以及有机催化的对醌与缺电子烯烃的分子间[5 + 2]环加成,构建asperones a(1)和B(2)的关键[3.2.1]辛烷值核心。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


