DUF4297 and HerA form abortosome to mediate bacterial immunity against phage infection
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Immune receptors form higher-order complexes known as inflammasomes in animals and resistosomes in plants to mediate immune signaling. Here, we report a similar bacterial protein complex, DUF4297-HerA, which induces abortive infection to mediate anti-phage immunity by coupling nuclease and ATPase activities. Therefore, we name this defense system “Hailibu” after a hunter in a popular folk tale who sacrifices himself to save his village. Cryoelectron microscopy (cryo-EM) results reveal that DUF4297 and HerA assemble into a higher-order complex, reminiscent of apoptosome, inflammasome, or resistosome, which we refer to as an abortosome. By capturing cryo-EM structures of the pre-loading, DNA-loading, and DNA-transporting states during Hailibu abortosome processing of DNA, we propose that DNA substrates are loaded through the HerA hexamer, with adenosine triphosphate (ATP) hydrolysis providing the energy to transport DNA substrates to the clustered DUF4297 Cap4 nuclease domains for degradation. This study demonstrates the existence of analogous multiprotein complexes in innate immunity across the kingdoms of life.
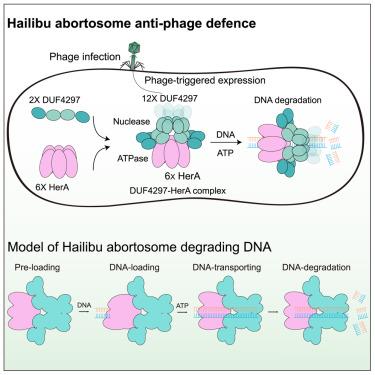
DUF4297和HerA形成流产体介导噬菌体感染的细菌免疫
免疫受体形成高阶复合物,在动物中称为炎症小体,在植物中称为抵抗小体,介导免疫信号。在这里,我们报道了一个类似的细菌蛋白复合物DUF4297-HerA,它通过偶联核酸酶和atp酶活性诱导流产感染介导抗噬菌体免疫。因此,我们将这种防御系统命名为“海力堡”,这是一个流行的民间故事中的猎人,他牺牲自己来拯救他的村庄。低温电子显微镜(cryo-EM)结果显示,DUF4297和HerA组装成一个高阶复合物,让人想起凋亡小体、炎症小体或抵抗小体,我们称之为流产小体。通过捕获海力步流产过程中预装载、DNA装载和DNA运输状态的低温电镜结构,我们提出DNA底物是通过HerA六聚体装载的,三磷酸腺苷(ATP)水解提供能量将DNA底物运输到DUF4297 Cap4核酸酶聚集区域进行降解。这项研究表明,在生命王国的先天免疫中存在类似的多蛋白复合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


