Ni(ii)-catalyzed enantioselective α-hydrazination of α-fluoroesters: access to chiral quaternary α-fluorinated α-amino acid derivatives†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d4qo02418d
引用次数: 0
Abstract
A highly catalytic asymmetric α-amination of α-azaaryl-α-fluoro esters with azodicarboxylates as the electrophilic aminating agents was reported. With C1-symmetric imidazolidine-pyrroloimidazolone pyridine as the tridentate ligand and Ni(BF4)2·6H2O as the Lewis acid, diverse chiral α-hydrazino-α-fluorinated ester derivatives were obtained in high yields (up to 97% yield) and excellent enantioselectivities (up to 98% ee).
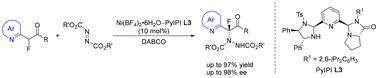
Ni(II)-催化α-氟酯的对映选择性α-肼化:手性季α-氟化α-氨基酸衍生物的制备
以偶氮二羧酸盐为亲电胺化剂,对α-氮杂杨基-α-氟酯进行了高催化不对称α-胺化反应。以c1对称咪唑烷-吡咯咪唑酮吡啶为三齿配体,Ni(BF4)2·6H2O为Lewis酸,以高收率(高达97%)和优异的对映选择性(高达98% ee)得到了手性α-肼基-α-氟化酯衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


