Identification of Covalent Cyclic Peptide Inhibitors Targeting Protein–Protein Interactions Using Phage Display
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
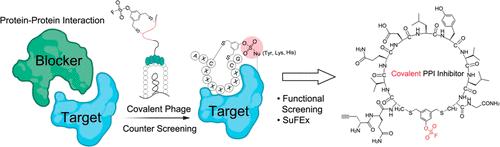
Peptide macrocycles are promising therapeutics for a variety of disease indications due to their overall metabolic stability and potential to make highly selective binding interactions with targets. Recent advances in covalent macrocycle peptide discovery, driven by phage and mRNA display methods, have enabled the rapid identification of highly potent and selective molecules from large libraires of diverse macrocycles. However, there are currently limited examples of macrocycles that can be used to disrupt protein–protein interactions and even fewer examples that function by formation of a covalent bond to a target protein. In this work, we describe a directed counter-selection method that enables identification of covalent macrocyclic ligands targeting a protein–protein interaction using a phage display screening platform. This method utilizes binary and ternary screenings of a chemically modified phage display library, employing the stable and weakly reactive aryl fluorosulfate electrophile. We demonstrate the utility of this approach using the SARS-CoV-2 spike-ACE2 protein–protein interaction and identify multiple covalent macrocyclic inhibitors that disrupt this interaction. The resulting compounds displayed antiviral activity against live virus that was irreversible after washout due to the covalent binding mechanism. These results highlight the potential of this screening platform for developing covalent macrocyclic drugs that disrupt protein–protein interactions with long lasting effects.
利用噬菌体展示技术鉴定靶向蛋白-蛋白相互作用的共价环肽抑制剂
肽大环由于其整体代谢稳定性和与靶标高度选择性结合相互作用的潜力,是一种很有前景的治疗多种疾病适应症的药物。在噬菌体和mRNA展示方法的推动下,共价大环肽的发现取得了最新进展,使得从各种大环的大型文库中快速鉴定出高效和选择性的分子成为可能。然而,目前用于破坏蛋白质-蛋白质相互作用的大环的例子有限,通过与目标蛋白质形成共价键起作用的例子就更少了。在这项工作中,我们描述了一种定向反选择方法,该方法能够使用噬菌体展示筛选平台识别针对蛋白质-蛋白质相互作用的共价大环配体。该方法利用二元和三元筛选化学修饰的噬菌体展示库,采用稳定和弱反应性的亲电试剂氟硫酸芳基。我们利用SARS-CoV-2刺突- ace2蛋白-蛋白质相互作用证明了这种方法的实用性,并鉴定了破坏这种相互作用的多种共价大环抑制剂。所得到的化合物对活病毒显示出抗病毒活性,由于共价结合机制,这种活性在冲洗后是不可逆的。这些结果突出了该筛选平台开发共价大环药物的潜力,这些药物可以破坏具有持久效果的蛋白质-蛋白质相互作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


