Dynamic In Vivo Mapping of the Methylproteome Using a Chemoenzymatic Approach
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
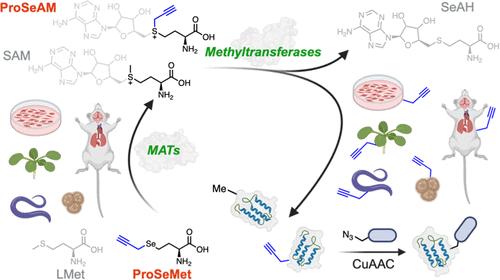
Dynamic protein post-translational methylation is essential for cellular function, highlighted by the essential role of methylation in transcriptional regulation and its aberrant dysregulation in diseases, including cancer. This underscores the importance of cataloging the cellular methylproteome. However, comprehensive analysis of the methylproteome remains elusive due to limitations in current enrichment and analysis pipelines. Here, we employ an l-methionine analogue, ProSeMet, that is chemoenzymatically converted to the SAM analogue ProSeAM in cells and in vivo to tag proteins with a biorthogonal alkyne that can be directly detected via liquid chromatography and tandem mass spectrometry (LC-MS/MS), or functionalized for subsequent selective enrichment and LC-MS/MS identification. Without enrichment, we identify known and novel lysine mono-, di-, and tripargylation, histidine propargylation, and arginine propargylation with site-specific resolution on proteins including heat shock protein HSPA8, the translational elongation factor eEF1A1, and the metabolic enzyme phosphoglycerate mutase 1, or PGAM1, for which methylation has been implicated in human disease. With enrichment, we identify 486 proteins known to be methylated and 221 proteins with novel propargylation sites encompassing diverse cellular functions. Systemic ProSeMet delivery in mice propargylates proteins across organ systems with blood–brain barrier penetrance and identifies site-specific propargylation in vivo with LC-MS/MS. Leveraging these pipelines to define the cellular methylproteome may have broad applications for understanding the methylproteome in the context of disease.
使用化学酶方法的甲基蛋白质组动态体内定位
动态蛋白翻译后甲基化对细胞功能至关重要,甲基化在转录调控及其在包括癌症在内的疾病中的异常失调中发挥重要作用。这强调了对细胞甲基蛋白质组进行编目的重要性。然而,由于目前富集和分析管道的限制,甲基蛋白质组的综合分析仍然难以捉摸。在这里,我们使用了l-蛋氨酸类似物ProSeMet,它在细胞和体内被化学酶转化为SAM类似物ProSeAM,用双正交炔标记蛋白质,可以通过液相色谱和串联质谱(LC-MS/MS)直接检测,或者功能化用于随后的选择性富集和LC-MS/MS鉴定。在没有富集的情况下,我们鉴定了已知的和新的赖氨酸单、二和三酰化、组氨酸丙氨酸化和精氨酸丙氨酸化,这些蛋白具有位点特异性分辨率,包括热休克蛋白HSPA8、翻译延伸因子eEF1A1和代谢酶磷酸甘油突变酶1 (PGAM1),甲基化与人类疾病有关。通过富集,我们鉴定出486种已知的甲基化蛋白和221种具有新的丙基化位点的蛋白,这些位点包括不同的细胞功能。ProSeMet在小鼠体内的全身传递使跨器官系统的蛋白质具有血脑屏障外显性,并通过LC-MS/MS鉴定体内特定位点的丙基化。利用这些管道来定义细胞甲基蛋白质组可能对理解疾病背景下的甲基蛋白质组具有广泛的应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


