meta-Nitration of Pyridines and Quinolines through Oxazino Azines
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
meta-Nitration of azines (pyridines and quinolines) serves as a powerful method for the prompt construction and derivatization of several pharmaceuticals, agrochemicals, and materials. However, due to the inherent electronic properties of pyridines, achieving direct selective meta–C–H nitration under mild conditions has been a long-standing challenge in synthetic chemistry. Currently, there is no adequate strategy for late-stage meta–C–H nitration of pyridine-containing drugs and drug precursors. To address this void, we introduce a practical protocol for the highly regioselective meta-nitration of pyridines using a dearomatization-rearomatization strategy. The introduced method provides a diversification platform for selective nitration at the meta-position of azines via a radical pathway. This mild, open-air, one-pot, scalable, and catalyst-free process is employed for the late-stage meta-nitration of pyridine containing drugs, drug precursors, and ligands using pyridines as the limiting reagents. Consecutive C3 and C5 difunctionalization of pyridines is also achieved with complete regiocontrol relying on sequential addition, which further highlights the potential of the presented work. Additionally, the obtained products could be transformed into meta-amino azine products and other valuable building blocks. Incorporating N-heterocyclic amine structures through amidation into ibuprofen has significantly improved the drug’s clinical success, highlighting the importance of this work.
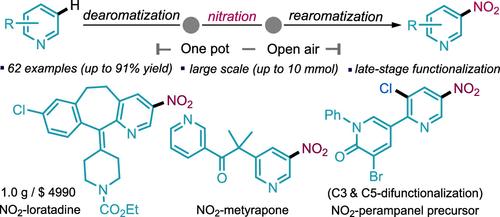
偶氮(吡啶和喹啉)的元硝化是一种快速构建和衍生多种药物、农用化学品和材料的有效方法。然而,由于吡啶固有的电子特性,在温和条件下实现直接选择性元-C-H 硝化一直是合成化学中的一个长期挑战。目前,还没有适当的策略用于含吡啶药物和药物前体的后期元-C-H 硝化反应。针对这一空白,我们介绍了一种利用脱芳烃-再芳烃化策略对吡啶进行高区域选择性元硝化的实用方案。所介绍的方法为通过自由基途径在叠氮的元位置进行选择性硝化提供了一个多样化的平台。这种温和、露天、一锅式、可扩展、无催化剂的工艺可用于以吡啶为限制试剂的含吡啶药物、药物前体和配体的后期元硝化。通过顺序加成,还实现了吡啶的连续 C3 和 C5 双官能化,并具有完全的区域控制能力,这进一步凸显了本研究成果的潜力。此外,获得的产物还可以转化为元氨基吖嗪产物和其他有价值的构筑物。通过酰胺化作用将 N-杂环胺结构加入布洛芬中,大大提高了该药物的临床疗效,凸显了这项工作的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


