Hydrophobic Ionic Liquid Engineering for Reversing CO Intermediate Configuration toward Ampere-Level CO2 Electroreduction to C2+ Products
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
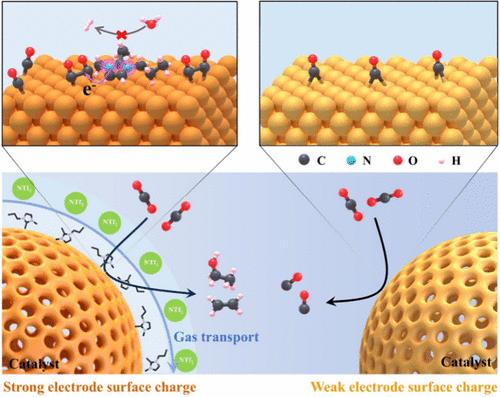
Hydrophobic ionic liquid (HIL) engineering on the catalyst surface represents a simple yet potent direction for optimizing the CO2 electroreduction performance. However, the pivotal role of HIL engineering at an industrial current density is still ambiguous due to limited and conflicting research findings. Herein, HIL-engineered oxide-derived Cu porous nanoparticles with electron-delocalized groups and a specific ultramicropore structure are first constructed to facilitate CO2-to-C2+ electroreduction at ampere-level current densities. The uniformly decorated HIL is innovatively demonstrated by positron annihilation lifetime spectroscopy, which offers unparalleled advantages in ultramicropore characterization. Bader charge-dependent performance analyses and theoretical calculations disclose that the N atoms in the HIL lower the adsorption energy of CO on the atop site from −0.38 to −1.42 eV through electron donation, which inverts the most stable adsorption site and favors the energy-efficient dimerization of atop-bound CO. Operando Raman spectra and in situ attenuated total reflection-surface enhanced infrared absorption spectroscopy indicate that the adhered HIL increases *CO coverage and alters the *CO adsorption configuration to an atop-bound state with an abundant high-frequency band. Furthermore, staircase potential electrochemical impedance spectroscopy unravels the specific arrangement structure of HIL enlarges the electrochemical surface charge by about 1.5 times, thereby accelerating CO2 electroreduction. As a result, the HIL-engineered oxide-derived Cu porous nanoparticles achieve a prominent C2+ productivity with a Faradaic efficiency of 85.1% and a formation rate up to 2512 μmol h–1 cm–2, outperforming most reported Cu-based electrocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


