Catalytic Regio- and Enantioselective Hydroformylation of Trisubstituted Alkenes to Construct α-Quaternary Lactams
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
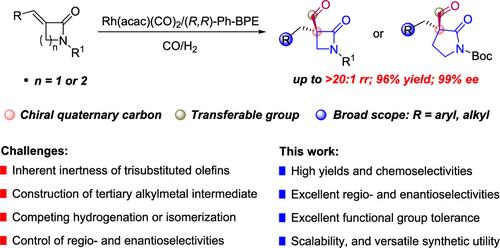
Developing novel stereoselective methods for α-quaternary lactams is of significant importance for advancing structure–activity studies, discovering new antibiotics, and synthesizing diverse functional compounds for synthetic and materials research. Herein, we have successfully developed a Rh-catalyzed asymmetric hydroformylation (AHF) of trisubstituted olefins, overcoming both the inherent inertness of trisubstituted olefins in such reactions and Keulemans’ rule, efficiently generating diverse β- and γ-lactams bearing an α-quaternary stereocenter with exceptional regio- and enantioselectivities (up to >20:1 rr, 99% ee). This mild and operationally simple reaction proceeds in an atom-economic manner with a broad substrate scope, along with excellent functional-group tolerance, scalability, and product diversification. Computational studies suggest that the enantio- and/or regioselectivity may originate from the Rh–C bonding along with the noncovalent interactions between the Boc group on the substrate and Ph groups on the ligand.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


