Electrochemical Lithiation Regulates the Active Hydrogen Supply on Ru–Sn Nanowires for Hydrogen Evolution Toward the High-Performing Anion Exchange Membrane Water Electrolyzer
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Designing a rational electrocatalyst/electrolyte interface with superb active hydrogen supply is of significant importance for the alkaline hydrogen evolution reaction (HER) and anion exchange membrane water electrolyzers (AEMWEs). Here, we propose a strategy to tune the interfacial active hydrogen supply via inducing dissoluble cation into electrocatalysts to boost HER in alkali, with electrochemical lithiated sub-2 nm RuSn0.8 nanowires (NWs) as a proof of concept. It is found that a part of Li+ could dissolve in situ from lithiated RuSn0.8 NWs during HER, which tends to affect the interfacial structure and facilitate the proton transport. Among all the Li–Ru–Sn and Ru–Sn NWs, the best-performing Li3.0RuSn0.8 NWs exhibit the lowest initial overpotential of 66 mV at 100 mA cm–2 in 1.0 M KOH, which could be further reduced to 38 mV after the 30 000 cycles accelerated stability test (AST). In situ Raman spectroscopy and operando X-ray adsorption spectroscopy indicate that the pristine Li3.0RuSn0.8 NWs are highly active toward water dissociation and the dissolved Li+ during AST could further enhance the flexibility of the hydrogen bond network for proton transportation. Ab initio molecular dynamics simulations and density functional theory calculations disclose that the incorporation of Li into the Ru–Sn lattice is beneficial to lower the water dissociation barrier, while dissolved Li+ at the interface significantly increases the population of interfacial water molecules, thereby providing sufficient active hydrogens for H2 production. The AEMWE equipped with the Li3.0RuSn0.8 NWs cathode delivers an extremely low cell voltage (1.689 V) at an industrial-scale current density (1 A cm–2) and outstanding stability (56 μV h–1 loss at 1 A cm–2 after 1000 h galvanostatic test), representing one of the best alkaline HER electrocatalysts ever reported.
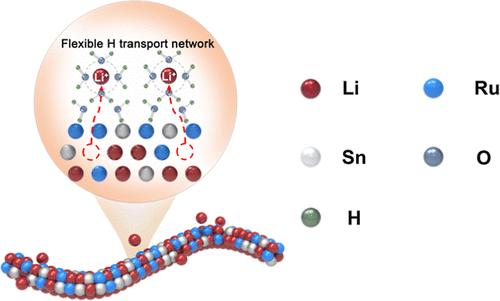
设计合理的电催化剂/电解质界面,使其具有极佳的活性供氢能力,对于碱性氢进化反应(HER)和阴离子交换膜水电解槽(AEMWEs)具有重要意义。在此,我们提出了一种通过诱导电催化剂中的可溶性阳离子来调节界面活性氢供应的策略,以电化学锂化的亚 2 纳米 RuSn0.8 纳米线 (NWs) 作为概念验证,从而促进碱中的氢进化反应。研究发现,锂化 RuSn0.8 纳米线中的部分 Li+ 可在 HER 过程中就地溶解,这往往会影响界面结构并促进质子传输。在所有锂-Ru-Sn 和 Ru-Sn NWs 中,性能最好的 Li3.0RuSn0.8 NWs 在 1.0 M KOH 中 100 mA cm-2 的初始过电位最低,为 66 mV,在经过 30 000 次加速稳定性测试 (AST) 后,过电位进一步降低到 38 mV。原位拉曼光谱和操作X射线吸附光谱表明,原始Li3.0RuSn0.8 NWs对水的解离具有很高的活性,AST期间溶解的Li+可进一步提高氢键网络在质子运输方面的灵活性。Ab initio 分子动力学模拟和密度泛函理论计算表明,在 Ru-Sn 晶格中加入 Li 有利于降低水解离障碍,而界面上溶解的 Li+ 能显著增加界面水分子的数量,从而为产生 H2 提供足够的活性氢。配备了 Li3.0RuSn0.8 NWs 阴极的 AEMWE 在工业规模的电流密度(1 A cm-2)下可提供极低的电池电压(1.689 V)和出色的稳定性(1000 h 电静态测试后,1 A cm-2 下的损耗为 56 μV h-1),是迄今报道的最佳碱性 HER 电催化剂之一。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


