Alkali Cation Inhibition of Imidazolium-Mediated Electrochemical CO2 Reduction on Silver
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
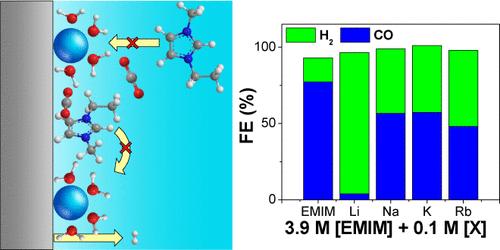
Imidazolium-based ionic liquids have led to enhanced CO2 electroreduction activity due to cation effects at the cathode surface, stabilizing the reaction intermediates and decreasing the activation energy. In aqueous media, alkali cations are also known to improve CO2 reduction activity on metals such as Ag, with the enhancement attributed to electrical double layer effects and trending with the size of the alkali cation. However, the effect of a mixed catholyte solution of alkali cations in the presence of an imidazolium-based ionic liquid has not been well-explored. Herein, 1-ethyl-3-methylimidazolium tetrafluoroborate, [EMIM][BF4], in water was investigated with alkali salts to unravel the interaction effects for CO2 electroreduction on Ag. Although both [EMIM]+ and alkali cations have individually improved CO2 to CO conversion on Ag in water, electrochemical results showed that alkali cations hindered imidazolium-mediated CO2 electroreduction in most conditions. Li+, in particular, was sharply inhibitory compared to other alkali cations and strongly redirected the selectivity to hydrogen evolution. The nature of the alkali cation inhibition was investigated with spectroscopic techniques, including in situ surface-enhanced Raman spectroscopy (SERS) and dynamic electrochemical impedance spectroscopy (DEIS). Along with computational insights from density functional theory (DFT), the electrochemical and spectroscopic data suggest that alkali cations inhibit [EMIM]-mediated CO2 reduction by competing for surface adsorption sites, preventing the potential-dependent structural reorientation of imidazolium, and promoting hydrogen evolution by bringing solvated water to the cathode surface.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


