Transition Metal Atoms Supported by a NbSSe Monolayer for Electrocatalytic Oxygen Reduction/Evolution Reactions
IF 3.3
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
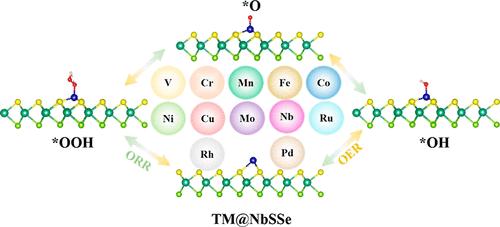
We suggest how to develop high-performance, low-cost, and stable oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) catalysts that may be relevant in the hydrogen economy. Transition metal dichalcogenides (TMDs) have great potential for electronics, photonics, catalysis, energy storage, and energy conversion due to their unique properties and structures. By using first-principles calculations, a series of single-atom catalysts (SACs) employing transition metal atoms adsorbed on a NbSSe monolayer are investigated jointly with the catalytic activity of TM@NbSSe for the ORR and OER. By stability and catalytic performance screening, Co@NbSSe is shown to have excellent ORR and OER activities irrespective of the Co atom being adsorbed on the S-edge or Se-edge of NbSSe, with the overpotential in the S-edge being 0.41 V (ORR) and 0.42 V (OER), while in the Se-edge, such values are 0.40 V (ORR) and 0.46 V (OER). It is also found that the Fe atom adsorbed on the Se-edge of NbSSe shows excellent bifunctional activity with overpotentials of 0.35 V (ORR) and 0.36 V (OER). Moreover, the Ni atom adsorbed on the S-edge of NbSSe showed a high OER performance with an overpotential of 0.46 V. Additionally, according to the linear relationship of intermediates, volcano plots are reported to describe the activity trends of the OER and ORR on TM@NbSSe. Scaling relationship between the d-band center and adsorption energy of the intermediates is also established to reveal the catalytic origin, with suitable d-band centers leading to moderate adsorption strength, all this being of relevance to attain good catalytic performances. Finally, the origin of the activity is elucidated by introducing as a descriptor the novel physical quantity φ, which is based on the intrinsic properties of the TM atoms.
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


