Crystallization Kinetics, Crystallization and Melting Lines of Deuterated and Hydrogenous Isotactic Polybutene-1
IF 5.1
1区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
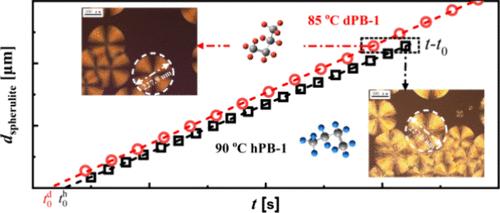
The kinetics of crystallization, lamellar long period, and melting temperatures (Tm) of hydrogenous isotactic polybutene-1 (hPB-1) and its fully deuterated counterpart (deuterated isotactic polybutene-1, dPB-1) with similar molecular weight at different isothermal crystallization temperatures (Tc) were investigated by means of fast scanning chip calorimetry and synchrotron microfocus small-angle X-ray scattering techniques. The Hoffman–Weeks plot where Tm was plotted as a function of Tc was used to determine the equilibrium melting point by extrapolating the data to Tm = Tc. Moreover, following the Gibbs–Thomoson equation and Strobl’s multistage crystallization model, the melting line and crystallization line where Tm or Tc is plotted as a function of inversed lamellar long period (1/dac) were constructed to determine the equilibrium melting temperature and equilibrium crystallization temperature by extrapolating the corresponding lines to infinite lamellar long period. The hPB-1 displays faster crystallization rates across the entire temperature range, suggesting a higher supercooling driving its crystallization than in dPB-1. However, hPB-1 possesses a lower equilibrium melting point/temperature but a higher equilibrium crystallization temperature than dPB-1. This peculiar isotope effect on the crystallization behavior of isotactic polybutene-1 provides a unique example supporting the crystallization mechanism proposed by Strobl where the supercooling with respect to the equilibrium crystallization temperature determines the crystallization kinetics.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Macromolecules
工程技术-高分子科学
CiteScore
9.30
自引率
16.40%
发文量
942
审稿时长
2 months
期刊介绍:
Macromolecules publishes original, fundamental, and impactful research on all aspects of polymer science. Topics of interest include synthesis (e.g., controlled polymerizations, polymerization catalysis, post polymerization modification, new monomer structures and polymer architectures, and polymerization mechanisms/kinetics analysis); phase behavior, thermodynamics, dynamic, and ordering/disordering phenomena (e.g., self-assembly, gelation, crystallization, solution/melt/solid-state characteristics); structure and properties (e.g., mechanical and rheological properties, surface/interfacial characteristics, electronic and transport properties); new state of the art characterization (e.g., spectroscopy, scattering, microscopy, rheology), simulation (e.g., Monte Carlo, molecular dynamics, multi-scale/coarse-grained modeling), and theoretical methods. Renewable/sustainable polymers, polymer networks, responsive polymers, electro-, magneto- and opto-active macromolecules, inorganic polymers, charge-transporting polymers (ion-containing, semiconducting, and conducting), nanostructured polymers, and polymer composites are also of interest. Typical papers published in Macromolecules showcase important and innovative concepts, experimental methods/observations, and theoretical/computational approaches that demonstrate a fundamental advance in the understanding of polymers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


