Manipulating Hydrogen-Bonding Donor/Acceptor in Ultra-Robust Isoreticular Zr(IV) Metal–Organic Frameworks for Efficient Regulation of Water Sorption Inflection Point and Steepness
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
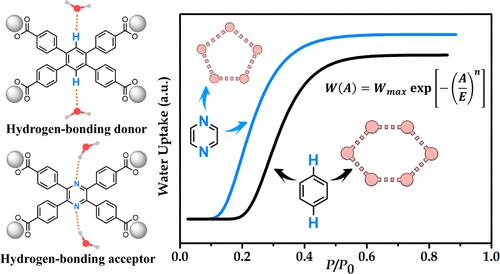
The development of porous materials exhibiting steep and stepwise adsorption of water vapor at desired humidity is crucial for implementing diverse applications such as humidity control, heat allocation, and atmospheric water harvesting. The precise molecular-level elucidation of structural characteristics and chemical components that dictate the water sorption behaviors in confined nanospaces, metal–organic frameworks (MOFs) in particular, is fundamentally important, but this has yet to be largely explored. In this work, by leveraging the isoreticular principle, we crafted two pairs of isostructural Zr-MOFs with linker backbones of benzene and pyrazine acting as hydrogen-bonding donor and acceptor, respectively. The outstanding water sorption cyclic durability of the four Zr-MOFs permits persuasive investigation of the correlation of the water sorption inflection point and steepness (the two central figures-of-merit for water sorption) with the linker functionality. The two pyrazine-carrying Zr-MOFs both show steep water uptake at lower relative pressure and slightly decreased steepness, which are quantitatively described by the Dubinin-Astakhov relation. We deciphered the privileged water clusters through single-crystal X-ray diffraction studies in which the pyrazine moiety formed stronger hydrogen-bonding interactions with guest water molecules and favored the formation of water pentamers instead of hexamers that are observed in the benzene analog. The hydrogen-bonding donor/acceptor manipulation approach presented in this work may facilitate future research endeavors focusing on molecular attribute engineering in predeterminedly ultrawater-resistant MOF platforms for efficient regulation of water sorption behaviors toward customized applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


