DNA Framework-Enabled Ocular Barrier Penetration for Microinvasive Antiangiogenic Therapy
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
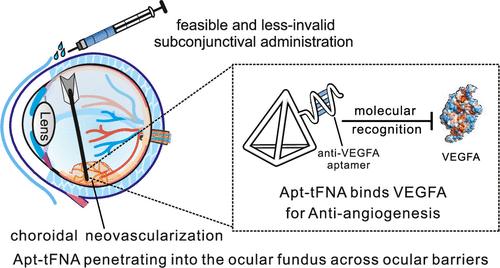
Therapeutic aptamers targeting vascular endothelial growth factor A (VEGFA) have advanced the development of antiangiogenic drugs for treating choroidal neovascularization (CNV) diseases. However, despite FDA approval for use in neovascular age-related macular degeneration (nAMD), the effective in vivo delivery of therapeutic aptamers is hindered by ocular barriers and rapid degradation in biofluids. Here, we demonstrated a microinvasive delivery of VEGFA-targeted aptamers to the ocular fundus using tetrahedral framework nucleic acids (tFNAs). Upon incorporating anti-VEGFA aptamers to the tFNAs (apt-tFNA), we interrogated their penetration across the outer blood–retinal barrier (oBRB) to the innermost retinal in the eyeball, while maintaining their structural integrity. In addition, the apt-tFNA showed superior efficacy in inhibiting vascular proliferation and migration by neutralizing VEGFA. Furthermore, in a laser-induced CNV mouse model, subconjunctival injection of apt-tFNA exhibited comparable antiangiogenic efficacy to intravitreal ranibizumab, a monoclonal antibody fragment. These findings suggest that FNAs can effectively deliver therapeutic aptamers to the ocular fundus without compromising their antiangiogenic properties, highlighting their potential for microinvasive and feasible periocular administration in treating neovascular ophthalmic diseases.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


