Pyridinium Rotor Strategy toward a Robust Photothermal Agent for STING Activation and Multimodal Image-Guided Immunotherapy for Triple-Negative Breast Cancer
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
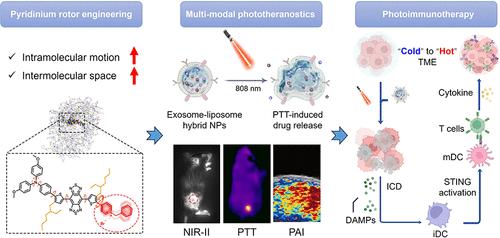
The immunosuppressive tumor microenvironment in triple-negative breast cancer could hinder the response to thorough immunotherapy and diminish the antitumor efficacy. Although the STING pathway emerges as a promising target to remedy defects, uncertain drug delivery might lead to off-target inflammatory reactions. Here, we manifest a novel phototheranostic agent with an aggregation-induced emission property that guided the pharmacological activation of a STING agonist for photothermal immunotherapy to create an immunologically “hot” tumor. A pyridinium rotor strategy is proposed to develop a positively charged TBTP-Bz, which is stably coincorporated with a STING agonist MSA-2 into thermal-responsive exosome-liposome hybrid nanoparticles for tumor-targeting delivery. TBTP-Bz exhibits aggregation-enhanced NIR-II emission and a photoacoustic signal, accomplishing real-time tumor tracking. Its photothermal stimulation induces immunogenic cancer cell death and promotes the precise release of MSA-2, thus boosting STING activation and STING-mediated type I interferon production. Significantly, single-dose photoimmunotherapy effectively suppresses abscopal tumor growth and provokes an immune memory effect to inhibit postsurgical recurrent and rechallenged tumors. This demonstrates promising clinical potential for poorly immunogenic breast cancer.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


