Porous and Amorphous MnxMo3S13 Chalcogel Electrode for High-Capacity Conversion-Based Lithium-Ion Batteries
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
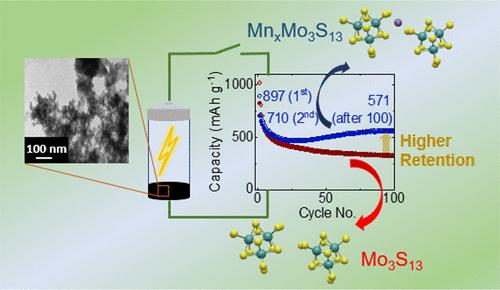
While Li-ion batteries (LIBs) are a leading energy storage technology, their energy densities are limited by the low capacity of conventional intercalation cathodes, driving interest in high energy-density Li–S batteries that make use of conversion chemistry. Achieving high capacity, reversibility, and cycle stability, and controlling volume changes in conversion batteries during the charge–discharge process, however, remains challenging. Here, we present a porous, amorphous, sulfide-based MnxMo3S13 chalcogel, which concurrently offers high capacity and cycle stability. The solution-processable room temperature synthesized MnxMo3S13 (x = 0.25) chalcogel exhibits a local structure that resembles the Mo3S13 cluster with Mn2+ distributed across the Mo3S13 matrix, as determined by synchrotron X-ray pair distribution function (PDF) and extended X-ray absorption fine structure (EXAFS). Ab initio molecular dynamics (AIMD) simulations reveal that Mn2+ incorporation shortens the polysulfide chain in the gel matrix compared to the Mo3S13 chalcogel, while forming a coordination environment with disulfide groups, analogous to the experimental findings. A Li/Mn0.25Mo3S13 half-cell delivers 897 mAh g–1 capacity during the first discharge and retains 571 mAh g–1 capacity after 100 cycles at a C/3 rate. Distribution of relaxation time (DRT) unveils a stable solid–electrolyte interphase (SEI) formation upon cycling that enables charge–discharge reversibility. Here, the enhanced capacity retention and cycle stability compared to those of the Li/Mo3S13 cell are attributed to the reduced dissolution of active mass into the electrolyte, facilitated by the formation of shorter polysulfide chains within the Mn0.25Mo3S13 structure and the strong affinity of Lewis-acidic Mn2+ for polysulfide anions generated during the charge–discharge process of the Li/Mn0.25Mo3S13 cell. Thus, this work illustrates a design principle of material for high-capacity and cycle-stable Li-metal sulfide batteries.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


