De Novo Design of Proteins That Bind Naphthalenediimides, Powerful Photooxidants with Tunable Photophysical Properties
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
De novo protein design provides a framework to test our understanding of protein function and build proteins with cofactors and functions not found in nature. Here, we report the design of proteins designed to bind powerful photooxidants and the evaluation of the use of these proteins to generate diffusible small-molecule reactive species. Because excited-state dynamics are influenced by the dynamics and hydration of a photooxidant’s environment, it was important to not only design a binding site but also to evaluate its dynamic properties. Thus, we used computational design in conjunction with molecular dynamics (MD) simulations to design a protein, designated NBP (NDI Binding Protein), that held a naphthalenediimide (NDI), a powerful photooxidant, in a programmable molecular environment. Solution NMR confirmed the structure of the complex. We evaluated two NDI cofactors in this de novo protein using ultrafast pump–probe spectroscopy to evaluate light-triggered intra- and intermolecular electron transfer function. Moreover, we demonstrated the utility of this platform to activate multiple molecular probes for protein labeling.
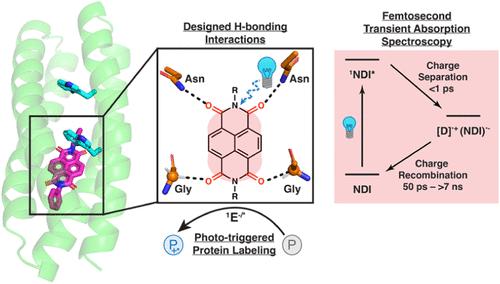
结合萘二亚胺的蛋白质从头设计,萘二亚胺是具有可调光物理性质的强光氧化剂
从头开始的蛋白质设计提供了一个框架来测试我们对蛋白质功能的理解,并构建具有自然界中未发现的辅助因子和功能的蛋白质。在这里,我们报道了设计用于结合强光氧化剂的蛋白质,并评估了使用这些蛋白质来产生可扩散的小分子反应物质。由于激发态动力学受光氧化剂环境的动力学和水合作用的影响,因此不仅要设计结合位点,而且要评估其动力学性质。因此,我们使用计算设计结合分子动力学(MD)模拟来设计一种蛋白质,命名为NBP (NDI结合蛋白),该蛋白质在可编程的分子环境中含有萘二亚胺(NDI),这是一种强大的光氧化剂。溶液核磁共振证实了该配合物的结构。我们使用超快泵浦探针光谱评估了该新生蛋白中的两个NDI辅助因子,以评估光触发的分子内和分子间电子传递函数。此外,我们证明了该平台的效用,以激活多个分子探针的蛋白质标记。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


