Synthesis of the tris-Amino Acid Labionin through a Contrasteric Aziridination and Ring-Opening Sequence
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
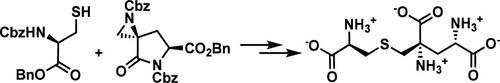
The non-proteinogenic tris-amino acid labionin was discovered in 2010 and since has been described in many natural products, defining an entire family of lanthipeptides. The unusual amino acid is biosynthetically produced by the sequential condensation of a cysteine onto two dehydroalanines. An attempt in 2011 to synthesize the amino acid monomeric unit was unsuccessful yet served as a valuable foundation and guide to the present work. A recently disclosed methodology for the selective opening of aziridines by mercaptan nucleophiles was applied to another methodology for contrasteric aziridination, thus enabling a short synthesis of challenging and elusive labionin.
通过异叠氮化和开环序列合成三氨基酸阴唇素
非蛋白性三氨基酸阴唇肽于2010年被发现,此后在许多天然产物中被描述,定义了整个蓝硫肽家族。这种不寻常的氨基酸是由半胱氨酸在两个脱氢丙氨酸上的连续缩合而产生的。2011年曾尝试合成氨基酸单体单元,虽然没有成功,但为目前的工作提供了有价值的基础和指导。最近公开的一种方法是巯基亲核试剂选择性打开叠氮嘧啶,该方法被应用于另一种方法,用于对比叠氮化,从而能够短时间合成具有挑战性和难以捉摸的阴唇素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


