Nucleic-acid-induced ZCCHC3 condensation promotes broad innate immune responses
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) and cyclic GMP-AMP synthase (cGAS) recognize aberrant nucleic acids and initiate antiviral responses. Host factor zinc finger CCHC domain-containing protein 3 (ZCCHC3) positively regulates both RLRs- and cGAS-mediated signaling through unknown mechanisms. Here, we show that ZCCHC3 employs a broad and unified strategy to promote these pathways in human cell lines. Rather than developing strong protein-protein interactions, ZCCHC3 harbors multiple nucleic-acid-binding modules and undergoes robust liquid phase condensation with nucleic acids. RNA-induced ZCCHC3 condensates enrich and activate RLRs, which then facilitate the interaction of RLRs with the downstream adaptor mitochondrial antiviral-signaling (MAVS). Direct and high-resolution structure determination of liquid condensates confirms the assembly of active-form MAVS filaments. Furthermore, ZCCHC3 efficiently promotes the condensation and enrichment of DNA, cGAS, ATP, and GTP, thereby enhancing cGAS signaling. ZCCHC3 mutants defective in RNA/DNA-induced condensation lost their regulatory efficiency in both pathways. These results highlight unexpectedly broad connections between biomolecular condensation and innate immunity.
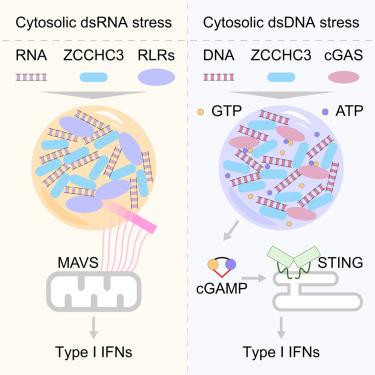
视黄酸诱导基因-I(RIG-I)样受体(RLRs)和环状 GMP-AMP 合成酶(cGAS)可识别异常核酸并启动抗病毒反应。宿主因子含锌指CCHC结构域蛋白3(ZCCHC3)通过未知机制积极调节RLRs和cGAS介导的信号传导。在这里,我们发现 ZCCHC3 采用了一种广泛而统一的策略来促进人类细胞系中的这些通路。ZCCHC3 并未发展出强大的蛋白质-蛋白质相互作用,而是蕴藏着多个核酸结合模块,并与核酸发生强有力的液相凝结。RNA 诱导的 ZCCHC3 凝聚物富集并激活 RLRs,然后促进 RLRs 与下游适配体线粒体抗病毒信号(MAVS)的相互作用。液态凝聚物的直接高分辨率结构测定证实了活性形式 MAVS 纤维的组装。此外,ZCCHC3 还能有效促进 DNA、cGAS、ATP 和 GTP 的凝聚和富集,从而增强 cGAS 信号转导。在 RNA/DNA 诱导的凝集方面存在缺陷的 ZCCHC3 突变体在这两种途径中都失去了调控效率。这些结果突显了生物分子缩聚与先天免疫之间意想不到的广泛联系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


