Bro̷nsted Acid-Catalyzed Reduction of Furans
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
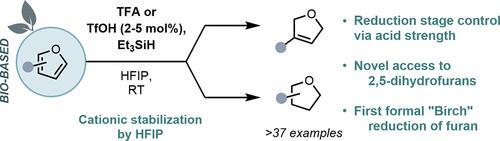
Bioderived furans play a pivotal role in advancing defossilized chemical pathways. The complete reduction of furans currently relies on impractical metal-catalyzed hydrogenations at high pressures and temperatures. In addition, the Birch reduction of unbiased furans to 2,5-dihydrofurans remains an unsolved synthetic challenge. Herein, we report a mild Bro̷nsted acid-catalyzed reduction of furans to 2,5-dihydro- and/or tetrahydrofuran derivatives using silanes as reducing agents. In particular, the first formal Birch reduction of furan itself is achieved. Mechanistic investigations reveal an intricate behavior of HFIP as the crucial solvent, preventing the intrinsic polymerization behavior of furans under acidic conditions and introducing additional driving force by specific product binding.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


