Biocatalytic atroposelective synthesis of heterobiaryls and heterobiaryl N-oxides via dynamic kinetic resolution†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-02-12
DOI:10.1039/d5qo00011d
引用次数: 0
Abstract
Heterobiaryl and heterobiaryl N-oxide atropisomers are important scaffolds in various chiral ligands, organocatalysts, and bioactive molecules. Here, we report a highly efficient biocatalytic route for the asymmetric synthesis of axially chiral heterobiaryl amines and heterobiaryl N-oxide amines via dynamic kinetic resolution (DKR). This novel DKR process features a racemization strategy promoted by forming a labile transition state via non-covalent interaction, coupled with a stereoselective reduction catalyzed by engineered imine-reductases (IREDs). Directed evolution of an IRED from Streptomyces sp. GF3546 provided two variants: S-IRED-Ss-M14 is superior for synthesizing diverse heterobiaryl amines, especially the ones containing multiple heteroatoms, and S-IRED-Ss-M16 is efficient for constructing heterobiaryl N-oxide amines. Both engineered IRED variants showed a broad substrate scope with a high level of yield and enantioselectivity (up to 98% yield and >99 : 1 enantiomeric ratio). This evolvable IRED-catalyzed DKR represents a promising solution for the atroposelective preparation of challenging axially chiral heterocyclic atropisomers.
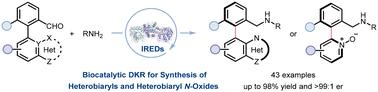
异芳基和异芳基n -氧化物的生物催化合成
杂冰芳基和杂冰芳基n -氧化物退聚体是各种手性配体、有机催化剂和生物活性分子的重要支架。本文报道了一种通过动态动力学分辨率(DKR)不对称合成轴手性杂芳基胺和杂芳基n -氧化物胺的高效生物催化途径。这种新的DKR过程的特点是通过非共价相互作用形成一个不稳定的过渡态来促进外消旋化策略,并通过工程亚胺还原酶(ired)催化立体选择性还原。从链霉菌(Streptomyces sp. GF3546)中定向进化的IRED有两个变体:S-IRED-Ss-M14擅长合成多种杂芳胺,特别是含有多个杂原子的杂芳胺;s- irred - ss - m16是一种高效的杂二芳基n -氧化物胺结构物。这两种改造的IRED变体均显示出广泛的底物范围、高产量和对映体选择性(高达98%的产量和>;99:1的对映体比)。这种可进化的ireds催化的DKR代表了一种有前途的解决方案,用于具有挑战性的轴手性杂环atropsiomer的制备。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


