One-Step HF-Free Synthesis of Alkali Metal Fluorides from Fluorspar
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
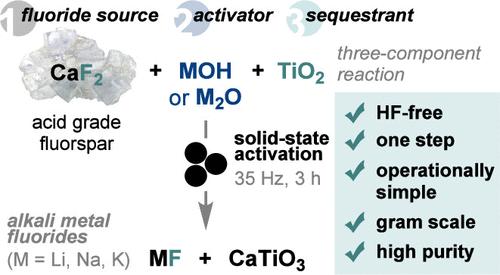
Alkali metal fluorides (MF) are commodity chemicals currently synthesized from naturally occurring fluorite (fluorspar, CaF2) in two steps: conversion of acid grade fluorspar (AGF) into highly hazardous hydrogen fluoride (HF) followed by neutralization with alkali metal hydroxides/carbonates. Herein, we report a one-step mechanochemical reaction that converts AGF into alkali metal fluorides under basic conditions, bypassing HF. The method consists of reacting AGF with alkali metal (hydr)oxides and titanium dioxide (TiO2) under mechanical energy for MF formation and in situ sequestration of calcium (hydr)oxide byproducts as calcium titanate (CaTiO3). Ca2+ sequestration prevents reversible CaF2 formation upon aqueous extraction, thus enabling the isolation of alkali metal fluorides. We also demonstrate that alkali metal titanates (M2TiO3) are suitable reagents for both CaF2 activation and Ca2+ sequestration, with K2TiO3 being optimal for KF synthesis.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


