Copper-Catalyzed Selective Amino-alkoxycarbonylation of Unactivated Alkenes with CO
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
1,2-Amino-difunctionalization reactions of alkenes allow the efficient introduction of different functional groups and the rapid construction of valuable functionalized amines. In this respect, we report a copper-catalyzed 1,2-amino-alkoxycarbonylation of unactivated alkenes with CO and alkylamine precursors in the presence of a Lewis acid additive. The novel protocol allows direct access to valuable β-amino acid derivatives from easily available starting materials. The presented methods feature high chemo- and regioselectivities, good functional group tolerance, and substrate scope including diverse bioactive compounds and drug-like molecules. Mechanistic studies indicate that the Lewis acid additive is the key to realizing the efficient umpolung addition of nucleophilic aminyl radicals to electron-rich alkenes, which represents an elegant activation strategy for aminyl radicals.
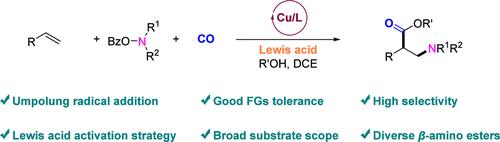
铜催化的未活化烯与 CO 的选择性氨基烷氧基羰基化反应
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


